Abstract
Enteric viruses, generally found in sewage, are recognized as the main cause of waterborne and foodborne public health outbreaks. Among leading enteric viruses, the Rotavirus A (RVA) detection in wastewater appeared to be a novel approach to monitor the emergence of these viruses in some countries where the viral gastroenteritis surveillance is almost absent such as in Tunisia. The RVA detection and quantification in an industrial sewage purification plant of Charguia I (Tunis, Tunisia) were achieved to evaluate the performance of activated sludge procedures coupled to a macrofiltration monolamp ultraviolet irradiation type C (UV-C254) disinfection reactor. This UV-C254 system was preceded by a fiberglass cartridge filter system with an average porosity of 45 μm to clarify the water and thus increase its UV transmittance. A total of 140 composite sewage samples was collected from this line of treatment and analyzed for RVA detection. The detection and the viral load quantification of RVA were performed using real-time reverse transcription polymerase chain reaction (RT-PCR). The virological results showed in general that RVA were detected at high frequency of 98% (137/140). In fact, the RVA detection rates at the exit of the two studied wastewater treatment were about 100% at the exit of the activated sludge procedure. It means that all wastewater sampled at this last step of treatment was positive for RVA detection. On the other hand, 92.5% of the wastewater samples taken at the exit of the monolamp UV-C254 reactor were positive for the RVA. However, the RVA quantification results expressed as viral load showed a significant reduction in the means of RVA viral loads at the exit of the biological activated sludge procedure and the tertiary UV-C254 treatment, showing in general an improved treated wastewater virological quality. Therefore, the RVA load removal rates recorded at the two successive stages of treatment, the activated sludge and the UV-C254 treatment, were around 85% and 73%, respectively, as compared to the one with 100% registered for the raw wastewater. In addition, good physical-chemical and bacteriological qualities of the treated sewage were found at the exit of the two considered wastewater treatment procedures. The present investigation represents the first Tunisian environmental report showing the good effectiveness and performance of the biological and the tertiary treatments for RVA removal. Therefore, an improved and an optimized tertiary disinfection treatment was needed since it could be a good means for getting better viral water quality and for minimizing the transmission and dissemination of human infectious viral diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rotaviruses group A (RVA) belonged to the Rotavirus genus, in the Sedoreovirinae subfamily and in the Reoviridae family (http://www.ictvonline.org/virusTaxonomy.asp) as it was reported in various scientific microbiological books such as Murray et al. (2016). RVA are small non-enveloped viruses, with an icosahedral capsid (Jayaram et al. 2004; Murray et al. 2016). The RVA genome is composed of ten double-stranded, positive sense, mono-cistronic RNA molecules and one double-stranded polycistronic RNA molecule coding for structural and non-structural proteins (Jayaram et al. 2004; Murray et al. 2016; Matthijnssens et al. 2011; Lever and Desselberger 2016; Desselberger 2014, 2017a). This group of viruses is divided into many serotypes based on the antigenic reactivity of the two-capsid structural proteins VP7 and VP4. The major glycoprotein VP7 and the minor protein VP4 encoded by gene 9, which defines 28 serotypes G (G1–G28) and by segment 4, that describes 39 serotypes P (P[1]-P[39]),respectively (Matthijnssens et al. 2008, 2012; Desselberger 2014, 2017a; Rega Institute, KU Leuven, Belgium 2017). The RVA is considered the first pathogenic etiological agent of acute viral gastroenteritis in pediatric populations (Murray et al. 2016; Estes and Greenberg 2013; Tate et al. 2016; Desselberger 2014, 2017a, 2017b). These viruses were recognized for their high genetic and antigenic assortment and diversity that are responsible of the emergence of some new genotypes every year (Trask et al. 2010; Navarro et al. 2013; Desselberger 2014, 2017a, 2017b). In different countries in the world, RVA was detected with significant rates in sewage samples collected in dissimilar wastewater treatment plants (Prevost et al. 2015; Ibrahim et al. 2016; Leifels et al. 2016; Motayo et al. 2016; Zhou et al. 2016; El-Senousy and Abou-Elela 2017; Assis et al. 2018). Thus, these viruses are considered emerging enteric viruses in aquatic environments. The monitoring of wastewater treatment plants is a suitable approach to study the RVA circulation at these particular sites. This allows for a better understanding of the molecular epidemiology of these viruses in children. In addition, the RVA are detected in various sewage purification plants and the resistance of these viruses to different wastewater treatment procedures in many countries such as France, Iran, Italy, Germany, Brazil, Tunisia, Egypt, Venezuela, China, and Nigeria (Rodriguez-Diaz et al. 2009; Kargaret al. 2013; Prevost et al. 2015; Ruggeri et al. 2015; Staggemeier et al. 2017; Ibrahim et al. 2016; Leifels et al. 2016; Motayo et al. 2016; Zhou et al. 2016; El-Senousy and Abou-Elela 2017; Assis et al. 2018).
The main objective of the present study was to improve the existing limited data on RVA detection rates and viral load values in sewage in Tunisia and to evaluate the performance of biological and tertiary wastewater treatment procedures for the removal of this type of gastroenteric virus from wastewater. This investigation, which has been conducted at the scale of a big industrial wastewater treatment plant of Charguia I (20,000 million cubic meters of treated wastewater per year), is considered the first study of its scale ever accomplished in Tunisia.
Materials and methods
Wastewater treatment plant and sewage sampling
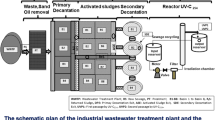
The present work was achieved at the scale of a big industrial sewage purification plant positioned in the business and residential region of Charguia I of Tunis City, northern Tunisia. This plant treats a wastewater flow of about 60,000 m3/day. The supplied wastewater is of different types such as domestic, hospital, urban, rain, and industrial coming from various areas of the great Tunis. The wastewater treatment plant Charguia I comprises successively four different treatment categories: the pre-treatment, the primary, the secondary or biological, and the tertiary wastewater treatment procedures (Fig. 1). Finally, the tertiary treatment was achieved by using a UV-C254 monolamp disinfection reactor. This reactor involves two stainless steel tanks, a cylindrical treatment chamber, and only one low-pressure germicidal lamp protected by a quartz sheath (wavelength = 253.7 nm, power = 55 W), a motor pump, a valve, and a cartridge fiberglass filter for ripening and clarification of secondary wastewater. The sewage to be purified circulates in the annular space between the quartz sheath and the inner wall of the irradiation chamber. A second passage of treated wastewater trough UV-C254 radiation is applied after a first one. Thus, treated wastewater passes twice through the irradiation chamber in order to raise the yield of the UV disinfection. The schematic plan of the industrial wastewater treatment plant Charguia I coupled with the UV-C254 disinfection reactor is illustrated in Fig. 1.
Throughout the 10 months of the present study (from June 2016 to April 2017), a total of 140 wastewater samples has been collected at various seasons and in the different sampling campaigns at the scale of the industrial Charguia I. Thus, at least seven sewage samples were collected every month as indicated: one sample at the entrance of wastewater treatment plant (WWTP) or raw wastewater (RW), one at the exit of primary treatment (OPT), one at the exit of activated sludge procedures (OAS), one at the exit of secondary treatment (OST), one sample at the exit of seeding sludge (SS), and at last, two samples after the first and the second passages through the UV-C254 irradiation chamber of the disinfection pilot reactor (UVP1, UVP2). As the last step, all treated wastewater mentioned as effluent of the plant will be stored in a big storage basin and the effluent will be canalized to the sea through a canal designed for this cause. This marine point of rejection of treated wastewater will be in the near future the subject of a follow-up study similar to the present study. The different sampling sites of wastewater were indicated and the monthly distribution of 140 wastewater samples was shown in Fig. 1 and Table 1.
Virological analyses
Virus concentration
Viruses were extracted from 1 L of wastewater, using the beef extract and AlCl3 upgraded technique (EPA 1992) in conformity with the US Environmental Protection Agency Protocol, as previously described by Ibrahim et al. (2015, 2016). The viral particles decontamination and precipitation were achieved using syringe filters (0.22 μm) (Sartorius, Germany) and the polyethylene glycol 6000 solution (PEG 6000, Bio Basic, Canada), respectively (Lewis and Melcaft 1988). The final volume, after the last step of viral particle concentration, was around 2 mL.
RNA extraction and real-time RT-PCR
The RVA genomic RNA was isolated from 800 μL of wastewater extract, using an automatic extraction instrument referenced NucliSENS® EasyMagTM platform (bioMérieux, Marcy L’Etoile, France) to obtain a final volume of 110 μL, according to the manufacturer’s instructions.
The RVA detection and quantification were achieved using real-time reverse transcription polymerase chain reaction (real-time RT-PCR). RVA was detected and quantified by five sense primers (Vp2-F1 to Vp2-F5); two antisense primers (Vp2-R1, Vp2-R2); and Vp2-Pprobe. The amplified sequences were those of the gene encoding for the RVA structural capsid protein (VP2) (Gutierrez-Aguirre et al. 2008; Ibrahim et al. 2016). All the reactions were accomplished using the TaqMan® Fast Virus 1-Step Kit (Applied Biosysytems) in a 7500 Fast Real-Time PCR thermocycler. RVA real-time RT-PCR reactions were made using the protocol described by Ibrahim et al. (2016). The RVA real-time RT-PCR was performed in two steps. In a first step, the denaturation of the RNA was carried out at 95 °C for 5 min in the presence of sterile water. The tubes were then put immediately at − 20 °C or on ice for at least 2 min. The reaction mixture of the real-time RT-PCR was also added to the RNA single-stranded molecules for the RVA quantification. In every reaction, a series of dilutions (100 to 109) was added in the RVA amplification and quantification that is obligatory for the measurement of the RVA viral load in sewage samples. The standard range of real-time RT-PCR was prepared using a plasmid R2237VP2cloneF/R clone. This plasmid derived from a cloned gene fragment of the major structural protein VP2 of RVA reference strain R2237, promoted by the National Reference Centre for enteric viruses in Dijon, France. A series of decimal dilutions ranging from 100 to 109 were prepared from this plasmid.
Physical-chemical and bacteriological analyses
The principal physical-chemical characteristics such as pH, temperature (T °C), suspended solids (SS), chemical oxygen demand (COD), and biological oxygen demand (BOD) were tested for all wastewater sampled at the entrance (RW) and at the exit (OST) of the WWTP Charguia I according to the Standard Methods for the Examination of Water and Wastewater (www.standardmethods.org/). In addition, the bacteriological analyses were achieved by the enumeration of the fecal coliforms (FC), fecal streptococci (FS), and Escherichia coli (EC) for all wastewater sampled at the entrance of WWTP (RW) and at the exit of every wastewater purification step (OPT, OAS, AS, SS, OST, UVP1, and UVP2), using the most probable number method (MPN) (www.standardmethods.org/).
Statistical analysis
Statistical analysis was achieved based on a one-way ANOVA using SPSS software (SPSS for windows version 22, Chicago, IL, USA). The mean values of the RVA viral load were compared by the least significant difference (LSD test), according to the post hoc of Student-Newman-Keuls test, at p < 0.05 and using the SPSS software program (SPSS for Windows, version 19; SPSS Inc., Chicago, IL, USA).
Results
Rotavirus A detection rates in sewage
It is important to note at the beginning of this paragraph that all the results obtained in this study were based on treatment effects by recording only the number of positive wastewater samples for rotavirus detection. Thus, the presence of infectious or non-infectious viral particles (active or not) does not arise since we have used only the RVA real-time RT-PCR technique (only technique available in our laboratory), which cannot differentiate between infectious and inactivated viral particles present in the wastewater. In fact, in this work, we measure only and strictly the effect of the treatment stage by only determining the presence or the absence of rotaviruses. Thus, a result in this study indicating a positive water sample for rotaviruses does not necessarily mean the presence of infectious particles; it can only be the simultaneous presence of both types of viral particles (inactivated and live virus particles). In addition, RVA results are discussed according to the average viral load registered in every step of treatment and expressed as genome copies per liter of water (gc/L).
Thus, the RVA detection results obtained in the present study showed that 98% of the collected samples from the biological and tertiary wastewater treatment procedures were positive for the RVA (137/140). In fact, 100 positive wastewater samples were from the various basins of activated sludge procedures (100/100 × 100 = 100%), and only 37 from the exit of the UV-C254 tertiary treatment (37/40 × 100 = 92.5%). The two-tertiary successive passages of UV disinfection gave the successive results (19/140 × 100 = 13.6%) for the first UV passage (UVP1) and (18/140 × 100 = 12.8% for the second (UVP2) passage, respectively. Moreover, the RVA circulation in the diverse basins, from the inflows to the outflows of the activated sludge procedure and in the UV-C254-treated effluents allowed to identify the detection rates of these viruses in the three treatment types as indicated, follows: 100% (n = 20/20) within the RW, 100% (n = 20/20) within the OPT, 100% (n = 20/20) within the SS, 100% (n = 20/20) within the OAS, 100% (n = 20/20) within the OST basin, and at last 95% (n = 19/20) and 90% (n = 18/20) within the exit of UVP1 and UVP2; respectively, the two-tertiary successive passage through UV-C254.
Change of the average RVA load (gc/L) according to the step of wastewater treatment
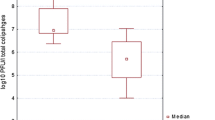
The RVA results showed that RVA were detected with higher and more significant frequencies in both raw wastewater (RW) and at the exit of the primary treatment (OPT) than those registered in the last three lagoon basins, such as the exit of activated sludge (OAS), the seeding sludge (SS) and the exit of secondary treatment (OST) of the activated sludge procedure. Indeed, the average RVA load has oscillated between 1.9 × 107 and 3.3 × 1010gc/L in the first two basins: the RW and OPT. However, the RVA median viral loads have varied between 1.8 × 107 and 2.8 × 109 gc/L in the last three basins OAS, SS, and OST. In addition, RVA was encountered in both RW and OPT basins at high frequencies with 100% in RW and in OPT, and with significant average viral loads of 5.8 × 109 gc/L in RW and 3.9 × 109 gc/L in the OPT, respectively. Similarly, the RVA detection rates were very high in the last three basins of activated sludge procedures and at the exit of the two successive UV-C254 passages. These frequencies were around 100% in the OAS, SS, and OST basins and around 90% in the first (UVP1) and the second (UVP2) UV-C254 passage. However, the average RVA load registered in the last three maturation basins of the biological wastewater treatment procedures (OAS, SS, OST) looked moderate as low if compared to those recorded in the first two basins of RW and OPT. These average loads were, respectively 5.8 × 108, 3 × 108, 5.1 × 108, 2.8 × 108, and 1.4 × 108 gc/L in the OAS, SS, and OST basins and at the exit of the first (UVP1) and the second UV-C254 (UVP2) passage, respectively. Consequently, the RVA load results in the three categories of treatment adopted in this study (primary, secondary, and tertiary) showed in general a significant reduction of the RVA average loads from the upstream to downstream of the activated sludge procedure, and at the exit of the two-successive UV-C254 passages. This reduction was observed from one type to another of the three different treatments adopted in this study (primary, biological, and tertiary treatments) (p ≤ 0:05) (Tables 2, 3; Fig 2). The RVA lessening rates between two successive basins were distributed in the activated sludge procedure as follows: 33% between the first two basins RW and OPT, 85% between OPT and OAS basins, 48.4% between OAS-SS basins, and finally, 12% between OAS-OST basins. Similarly, the RVA rates at the exit of the two successive UV-C254 passages UVP1 and UVP2 were about of 45.4 and 73%, respectively.
Abatement of the main physical-chemical and bacteriological parameters
The physical-chemical and bacteriological results in the present study demonstrated a substantial decrease of the median values of the main parameters tested from upstream to downstream of the studied biological wastewater treatment procedure of WWTP Charguia I. In fact, the mean values of the major physical-chemical parameters studied decreased from 207 to 17.3 mg/L for suspended solids (SS), from 576 to 76.7 mg O2/L for chemical oxygen demand (COD) and from 330 to 17.7 mg O2/L for biological oxygen demand (BOD5) (Table 3). Moreover, the concentrations of the fecal bacteria reduced from 8.4 × 104 to 16 × 103 MPN/100 mL for fecal coliforms (FC), 5 × 104 to 6 × 105 MPN/100 mL for fecal streptococci (FS), and 17.2 × 104 to 7 × 104 MPN/100 mL for Escherichia coli (EC). Consequently, the obtained results showed the effectiveness and the performance of activated sludge procedures for the physicochemical and bacteriological pollution abatement recording thus a high reduction rate of the main parameters tested (95% for BOD5, 87% for COD, and 92% for SS; 98% for FS, 88% for FC, and 96% for EC). All the mean values of the physico-chemical characteristics obtained at the exit of the activated sludge procedure (OST) in this study are lower than those set by the Tunisian wastewater discharge standard (ST 106-02) (SS = 30 mg/L; COD = 90 mg O2/L; BOD5 = 30 mg O2/L). Nevertheless, the average content of these fecal bacteria remaining at the exit of the biological treatment procedures are very important as compared to those fixed by the Tunisian wastewater discharge standard (ST 106-02) (FC = 2 × 103 MPN/100 mL; FS = 103 MPN/100 mL). The tertiary treatment results showed a substantial reduction of the median concentration of these fecal bacteria after the two successive passages through the UV-C254 reactor. Indeed, these concentrations decreased from 16 × 103 to 0.3 MPN/100 mL for FC, from 6 × 105 to 0.3 MPN/100 mL for FS, and from 7 × 104 MPN/100 mL to 0.3 MPN/100 mL. In addition, these values were consistent with the Tunisian standards of wastewater discharge (ST 106-02) (FC = 2 × 103 MPN/100 mL, FS = 103 MPN/100 mL) and the abatement rates of these bacteria between the biological and tertiary treatment are similar, they were 99.9% or 3 U log. Therefore, these findings showed the effectiveness and the performance of UV-C254 monolamp disinfection reactors intended for the fecal bacteria reduction.
Seasonal distribution of positive rotaviruses A in wastewater samples
The obtained results of the RVA mean viral loads were used to assess the seasonality distribution of this type of enteric viruses in the 140 wastewater samples collected from WWTP Charguia I during the 10 months of study (from June 2016 to April 2017). The monthly distribution of RVA frequencies showed that these viruses were detected continuously in circulated sewage with an alkaline pH (7.8–8.3) and ambient temperatures varying between 10 and 40 °C throughout the sampling period, and with a high RVA detection rate in all seasons. Indeed, the RVA frequencies were distributed as follows: 71–100% in summer, 95–100% in autumn, and 100% in winter and spring. However, the RVA quantification results demonstrated that these viruses were detected with low to moderate average frequencies during the dry period (June to November months) and with some significant median viral frequencies during the rainy period (December to March months), thus recording an important peak during winter and spring (Table 2; Fig. 3). The average viral load of RVA has oscillated between 1.8 × 109 and 7.1 × 109 gc/L during the cold seasons (Table 2; Fig. 3). Nevertheless, the median RVA load has fluctuated between 3.5 × 107 and 8.1 × 108 gc/L during the hot seasons (Table 2; Fig. 3). In addition, RVA was encountered along the four seasons with a notable peak during winter and spring in the first two basins (RW and OPT) of the activated sludge procedure.
Discussion
In the present report, the virological results showed that RVA was detected in 98% of the wastewater sampled at the scale WWTP Charguia I positioned in a residential and business area of Tunis City, northern of Tunisia. The primary environmental study conducted by Ibrahim et al. (2016) reported that the RVA detection rate of around 50% of the sewage collected at a pilot WWTP located in a residential and hospital area of Tunis City, northern of Tunisia. Consequently, the results of this study let to show a significant increase of the RVA detection frequency in the wastewater draining in this same area of study. Indeed, these frequencies increased from 50% in a pilot WWTP to 98% in the industrial WWTP of Charguia I. The important increase of RVA detection rates recorded in the two studies indicated the general emergence, development, and diffusion of this type of gastroenteric viruses commonly dispersed in sewage in the region of the city of Tunis. In addition, the RVA detection rate of 98% registered in the present study appeared as dissimilar to those shown in the two earlier Tunisian environmental studies (32–72%). These last two frequencies have been registered in wastewater samples collected at the scale of three different WWTP situated in the touristic area of Monastir, Sahel region of Tunisia (Sdiri-Loulizi et al. 2010; Hassine-Zaafrane et al. 2015). Therefore, these data also showed the substantial increase of RVA frequency between the two different regions. The important increase of RVA detection frequencies recorded in the three Tunisian environmental studies indicated and agreed on the effect the emergence and the diffusion of these types of gastroenteric virus in the sewage of Tunis and Monastir areas. The emergence and the great diffusion of this type of gastroenteric viruses in Tunisian wastewater is conditioned and related to many various factors associated with the social, hygienic, and geographical dissimilarities of each contact population.
Furthermore, the RVA detection rates recorded in the present work were similar to those reported in earlier and original environmental studies achieved in contaminated wastewater sampled from different wastewater treatment plants in diverse regions of the world. Likewise, RVA was detected with high frequencies in wastewater sampled in various WWTPs situated in China (93.5%), Italy (60.4%), New Caledonia (75%), Egypt (84.6%), and Uruguay (70%) (Zhou et al. 2016; Ruggeri et al. 2015; Kaas et al. 2016; El-Senousy and Abou-Elela 2017; Lizasoain et al. 2018). However, RVA were reported with low detection rates in sewage samples collected in different WWTPs located in Nigeria (14.2%) and in Germany (19%) (Leifels et al. 2016; Motayo et al. 2016). Many other environmental reports demonstrated that RVA frequencies were around of 73.33% and 100% in the inflows and of 26.67% and 21% in the outflows of the sewage sampled at the scale of different WWTPs in Iran and in France, respectively (Kargar et al. 2013; Prevost et al. 2015). In Brazil, the RVA detection rates oscillated between 12.3 and 95% in the collected wastewater samples (Miagostovish et al. 2008; Prado et al. 2011; Fumian et al. 2011; Staggemeier et al. 2017; Assis et al. 2018). Therefore, the high RVA frequency rate confirmed that these viruses are recognized as emergent gastroenteric viruses in numerous natural aquatic environments and could be useful for the vaccine-monitoring program, the waterborne and foodborne disease prevention related with acute viral gastroenteritis.
Similarly, the RVA quantification results showed that these gastroenteric viruses were detected in WWTP Charguia I with significant average viral loads. These average loads were around 5.8 × 109 gc/L in RW and 5.1 × 108 gc/L in OST, and 1.4 × 108 gc/L at the exit of UV-C254 treatment (UVP2). This first Tunisian environmental study in the northern area of Tunisia reported that the RVA quantification loads were on average more and less low to moderate from the first to the last basins of natural lagoon procedures and at the exit of a rotating biodisks process in a pilot WWTP. These loads have oscillated between 125 and 11 gc/μL (Ibrahim et al. 2016). Therefore, these data indicated an important average viral load of RVA circulating in the sewage in this area of the city of Tunis.
Consequently, the important RVA average viral load of around 5.8 × 109 (gc/L) and the high frequency of positive wastewater samples for RVA, with around 98%, registered in the present study confirmed the increase of foodborne and waterborne outbreaks related to viral gastroenteritis in these areas that lack enteric virus epidemic surveillance. Subsequently, these findings established that sewage monitoring constitutes an original approach for the waterborne prevention and anticipation of viral pandemic gastroenteritis.
In addition, these last findings were dissimilar from those described in many environmental studies conducted in various countries of the world such as France, Brazil, Egypt, China, and Uganda (Prevost et al. 2015; Staggemeier et al. 2017; El-Senousy and Abou-Elela 2017; Zhou et al. 2016; O’Brien et al. 2017). In fact, in all these environmental studies, the recorded average viral loads of RVA were much higher than the ones registered in the present study. Indeed, the RVA average loads have fluctuated in sewage from 102 to 105 gc/L in France, from 103 to 109 gc/L in Brazil, 1.1 107 to 2.4 107 gc/L in Egypt, 1.5 103 to 1.3 106 gc/L in China, and at last from 5.79 101 to 3.77 103 gc/L in Uganda (Prevost et al. 2015; Staggemeier et al. 2017; El-Senousy and Abou-Elela 2017; Zhou et al. 2016; O’Brien et al. 2017; Assis et al. 2018).
The RVA detection and quantification at the input and at the output of the three different wastewater treatment procedures (primary decantation, activated sludge, UV-C254) are conducted in the WWTP Charguia I for the determination and the evaluation of the performance of these purification procedures in removing this type of virus. Thus, the results of RVA quantification in the present study showed a significant decrease of the average viral load of RVA in the treated wastewater by activated sludge and UV-C254 procedures. The RVA average viral loads declined from 5.8 × 109 gc/L in raw wastewater (RW) to 5.1 × 108 gc/L in the output of the secondary biological wastewater treatment procedure (OST). In addition, the RVA average viral loads showed a moderate reduction from 5.1 × 108 gc/μL at the exit of the secondary treatment (OST) to 1.4 × 108 gc/L at the exit of the second UV-C254 passage (UVP2). Therefore, the RVA detection results showed no significant difference in the frequencies of these viruses registered in the different basins of the activated sludge and after the two successive passages through UV-C254 treatment procedures. The RVA was found with high and similar frequencies in raw wastewater (100%) and in the purified effluents by the implemented biological (100% in OST) and tertiary (90% in UVP2) wastewater treatment procedures. Based on the RVA average viral loads registered across the line of the activated sludge procedures indicated that this type of gastroenteric viruses was lowered with an average removal efficiency of around 91% in the case of the activated sludge process and around 73% in the case of the second step of UV-C254. Thus, the biological appeared as more efficient and effective concerning the reduction of the average viral load as compared to the one determined in the case of the second step of UV-C254. According to these last results obtained and mentioned above, it appeared that wastewater treatment by activated sludge was more effective than the tertiary treatment using the disinfection UV-C254 system concerning the abatement of the viral particles. Indeed, the treatment by disinfection is in general a complementary treatment and it is not necessarily more efficient than the one obtained by the activated sludge process. The intrinsic biophysicochemical conditions of wastewater are an important and decisive factor in the determination of the output of disinfection. Thus, these conditions are, in general, more stressing for the survival and the maintenance of viral particles in charged water than in clear water. In the same way, there would be many viral particles that would be eliminated following their adsorption to the various suspended matter and solids and they would be eliminated during the process of primary and secondary wastewater settling or clarification.
Therefore, these data established an improvement of the virological quality of the purified effluents intended for recycling, agriculture reuse, and discharges into natural aquatic receiving environments. Consequently, the epidemiology monitoring systems of RVA circulating in polluted wastewater will be advantageous for the improvement of the virological quality of purified effluents. In addition, the obtained results in the present research work represent the first Tunisian environmental report showing the performance of the biological (activated sludge procedures), and particularly tertiary sewage purification procedures (UV-C254 disinfection) for RVA removal. Despite the high effectiveness and performance of the two studied wastewater treatment procedures, the RVA looked circulating with important frequencies (100% in OST basins; 90% in UVP2) but with average low contents (5.1 × 108 and 1.4 × 108 gc/L in OAS and UVP2, respectively) in the purified effluents intended for agronomic reuse, industrial recycling, and release in the natural aquatic receiving environment.
These findings were similar to earlier first two Tunisian environmental reports conducted at the pilot WWTP El Menzeh I situated in the same area of the present study, north of Tunisia. These last two studies showed a moderate effectiveness of two biological or secondary sewage purification procedures: rotating biodisks and natural lagoons for Noroviruses GII and RVA removal. The abatement rates of these viruses were about of 50% for RVA and 54.8% for Noroviruses GII (Ibrahim et al. 2015, 2016). Moreover, a recent Egyptian environmental study reported by El-Senousy and Abou-Elela (2017) demonstrated the effectiveness of two biological wastewater treatment procedures: anaerobic sludge blanket (UASB) and biological aerated filters (BAF) for enteric virus removal such as rotaviruses, noroviruses GI, GII, adenoviruses, and hepatitis E viruses. Nevertheless, two earlier Tunisian environmental studies conducted in various WWTPs situated in the Monastir touristic area, Sahel of Tunisia, reported the ineffectiveness of activated sludge procedures for noroviruses GI, GII, RVA, and sapoviruses abatement and reduction (Sdiri-loulizi et al. 2010; Hassine-Zaafrane et al. 2014, 2015; Varela et al. 2018). In addition, the findings of this study were dissimilar from those reported in other recent Tunisian environmental studies also describing the ineffectiveness of the natural oxidation ponds and rotating biodisks in the pilot WWTP El Menzeh I of Tunis City for aichivirus, astrovirus and adenovirus removal (Ibrahim et al. 2017a, 2017b, 2018). In addition, a French environmental study indicated the ineffectiveness of activated sludge and biological filtration procedures for enteric virus removal, case of RVA (Prevost et al. 2015). Furthermore, two other same studies conducted in Uruguay and Canada mentioned the high resistance of RVA to various biological and tertiary wastewater treatment procedures, such as activated sludge, ultraviolet disinfection, membrane ultrafiltration (UF) and chlorine (Cl2) (Qiu et al. 2015; Lizasoain et al. 2018). Moreover, the resistance of RVA to biological sewage purification procedures such as trickling filters and activated sludge was reported, recording a low removal efficiency of around 0.76–0.99 annual average log10 reduction (Tonani et al. 2013; Kitajima et al. 2014).
In addition, the different physico-chemical and bacteriological analysis results indicated a substantial reduction of the average value of the major parameters tested (SS, BOD5, COD) and an important decrease of the fecal bacteria (FC, FS, E. coli) from the upstream to downstream of the biological wastewater purification procedure. Indeed, the average value of the physico-chemical characteristics at the exit of the secondary activated sludge treatment (OST) appeared lower than those recommended by the Tunisian standard (ST 106-02) of wastewater discharges. Thus, the average contents of the fecal bacteria at the exit of the activated sludge procedures (OST) appeared higher than the ones fixed by the Tunisian standard (ST 106-02) (FS = 6 × 105 > to 103, FC = 16 × 103 > to 2 × 103). Consequently, these data indicated a good physicochemical sewage quality of the purified effluent and a bad bacteriological quality of this effluent treated by the activated sludge procedures. Thus, the effects of the UV-C254 treatment are crucial and decisive since a tertiary treatment is required for the elimination of these fecal pathogenic bacteria circulating in the purified effluents, which may establish and constitute important menaces and dangers for public health and the natural environment in general. These findings were in accordance with those described in other studies showing the high performance of activated sludge procedures as for the physicochemical abatement (3 U logs) and the moderate effectiveness of this biological purification for the bacteriological abatement (1.5–2 U logs) (Campos et al. 2016 ; Dai et al. 2016; Sano et al. 2016; Grandclément et al. 2017). Nevertheless, another earlier Italian environmental report conducted by La Rosa et al. (2010) showed that the removal efficiency was around 99% (2 U logs) for fecal indicator bacteria. In addition, these data were in agreement with those reported in other Tunisian environmental studies in the same area showing the ineffectiveness of a rotating biological disk for the fecal bacteria indicator removal (Ibrahim et al. 2015, 2016, 2017a, 2017b, 2018). However, the results of the UV-C254 tertiary treatment showed an excellent bacteriological quality of the purified effluents; with average fecal bacteria contents much lower than the ones set by the Tunisian wastewater discharge standard (ST 106-02). Therefore, these data indicated that these purified sewage could be used for recycling, agriculture reuse, and harmless releases in natural aquatic environments. All these previous results are in accordance with other comparable reports showing and confirming the almost total elimination of pathogenic microorganisms, such as fecal bacteria and enteric viruses from the purified effluent by UV-C treatment (Qiu et al. 2015; Lizasoain et al. 2018). Similarly, the results obtained in our present study were concordant and similar to those obtained in previous studies conducted by Hassen et al. (2000), Ben Said et al. (2010), and Turki et al. (2017). These authors demonstrated the efficacy of tertiary UV-C254 treatment as for the several bacterial communities such as Pseudomonas aeruginosa, Salmonella, and Enterococci.
The RVA seasonal distribution during the 10 months of study, from June 2016 to April 2017, indicated the presence of this type of gastroenteric viruses with important detection rates during all months of the sampling period. These frequencies were around 100% in all months except October and June, where the frequencies were around 90% and 71%, respectively. Nevertheless, the seasonal distribution of RVA average viral load through the period of study was detected and quantified with significant seasonality recording thus important peaks during the cold and rainy months (winter and spring seasons), corresponding to the RVA seasonal peak loads and to the epidemic period of viral gastroenteritis in pediatric populations in Tunisia and other regions in the world (Sdiri-Loulizi et al. 2008; Pang et al. 2014; Desselberger 2014, 2017a). These mean viral loads varied between 1.7 × 109 and 7.1 × 109 gc/L in the cold seasons. These results obtained are in agreement with those described in earlier environmental studies conducted in Tunisia and in the same area of this study, similarly showing the norovirus GII, aichivirus, astrovirus, and enteric adenovirus abundance during the two seasons of winter and spring (Ibrahim et al. 2017a, 2017b, 2018). Likewise, these findings were in accordance with those described in other environmental studies conducted in southern Tunisia, Canada, France, Uganda, and China, which showed that these viruses are the most prevalent during winter and spring (Sdiri-Loulizi et al. 2010; Qiu et al. 2015; Prevost et al. 2015; O’Brien et al. 2017; He et al. 2011). However, our data are in discordance with other environmental studies conducted in Brazil and in the USA reporting that RVA were detected in wastewater samples without seasonal variation during the sampling period (Tonani et al. 2013; Kitajima et al. 2014; Assis et al. 2018).
Conclusion
This study highlighted the high detection rates and the important average viral loads of RVA in inflows and outflows of a wastewater treatment plant, positioned in the residential and industrial area of Charguia I of Tunis City, northern Tunisia. In addition, the present report established the high performance and effectiveness of the assumed biological wastewater treatment procedure in Tunisian treatment plants using activated sludge procedures for the fecal coliforms, fecal streptococci, E. coli, and the RVA removal, presenting a good bacteriological and virological quality of the purified effluents. Despite the effectiveness of the activated sludge procedure for the enteric pathogen abatement, fecal bacteria and RVA were still circulating in the treated effluents, which constitute a risk for agriculture, animal, and human populations. The tertiary treatment results showed that an excellent bacteriological quality and almost total disinfection of the purified effluents were obtained at the exit of the second passage by UV-C254 monolamp reactors. However, a moderate improvement of the virological quality of the purified effluents was acquired by UV-C254 treatment. Therefore, this data demonstrated the insufficient sanitary quality of treated wastewater envisioned for agricultural reuse, recycling, and releases into natural aquatic environments. The tertiary treatment by ultraviolet irradiation seemed necessary in all Tunisian wastewater treatment plants in order to upgrade the microbiological quality of the treated effluents by biological purification. These findings reflect the epidemiology of these viruses in the pediatric population, highlighting that treated wastewater establishes a route of RVA spread and a source of viral pandemic gastroenteritis related to waterborne and foodborne epidemics.
References
American Public Health Association (2017) Standard methods for the analysis of water and wastewater, Joint Publication of the American Public Health Association, American Water Works Association, and Water Environment Federation. Second Edition. Washington, DC, USA, 2012. Http: www.standardmethods.org/. Accessed 27 November 2017.
Assis ASF, Fumian TM, Miagostovich MP, Drumond BP, da Rosa E, Silva ML (2018) Adenovirus and rotavirus recovery from a treated effluent through an optimized skimmed-milk flocculation method. Environ Sci Pollut Res Int 25(17):17025–17032. https://doi.org/10.1007/s11356-018-1873-x
Ben Said M, Masahiro O, Hassen A (2010) Detection of viable but non-cultivable Escherichia coli after UV irradiation using a lytic Qbeta phage. Ann Microbiol 60(1):121–127. https://doi.org/10.1007/s13213-010-0017-4
Campos CJA, Avant J, Lowther J, Till D, Lees DN (2016) Human norovirus in untreated sewage and effluents from primary, secondary and tertiary treatment processes. Water Res 103:224–232. https://doi.org/10.1016/j.watres.2016.07.045
Dai H, Chen W, Lu X (2016) The application of multi-objective optimization method for activated sludge process: a review. Water Sci Technol 73(2):223–235. https://doi.org/10.2166/wst.2015.489
Desselberger U (2014) Rotaviruses. Virus Res 190:75–96. https://doi.org/10.1016/j.virusres.2014.06.016
Desselberger U (2017a) Differences of rotavirus vaccine effectiveness by country: likely causes and contributing factors. Pathogens 6(4):65. https://doi.org/10.3390/pathogens6040065
Desselberger U (2017b) Reverse genetics of rotavirus. Proc Natl Acad Sci U S A. 114(9):2106–2108. https://doi.org/10.1073/pnas.1700738114
El-Senousy WM, Abou-Elela SI (2017) Assessment and evaluation of an integrated hybrid anaerobic-aerobic sewage treatment system for the removal of enteric viruses. Food Environ Virol 9(3):287–303. https://doi.org/10.1007/s12560-017-9286-4
EPA Environmental protection agency (1992) Standard for the disposal of sewage sludge. In Federal Register, part 503, pp 9387–9404
Estes MK, Greenberg HB (2013) Rotaviruses. In: Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (eds) Fields virology, 6th edn. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, PA, pp 1347–1401
Fumian TM, Leite JP, Rose TL, Prado T, Miagostovich MP (2011) One-year environmental surveillance of rotavirus specie A (RVA) genotypes in circulation after the introduction of the Rotarix® vaccine in Rio de Janerio, Brazil. Water Res 45(17):5755–5763
Grandclément C, Seyssiecq I, Piram A, Wong-Wah-Chung P, Vanot G, Tiliacos N, Roche N, Doumenq P (2017) From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: a review. Water Res 111:297–317. https://doi.org/10.1016/j.watres.2017.01.005
Gutierrez-Aguirre I, Steyer A, Boben J, Gruden K, Poljsak-Prijatelj M, Ravnikar M (2008) Sensitive detection of multiple rotavirus genotypes with a single reverse transcription-real-time quantitative PCR assay. J ClinMicrobiol 46(8):2547–2554. https://doi.org/10.1111/lam.12839
Hassen A, Mahrouk M, Ouzari H, Cherif M, Boudabous A, Damelincourt JJ (2000) UV Disinfection of treated wastewater in a large scale pilot plant ant inactivation of selected bacteria in a laboratory UV device. Bioress Technol 74(2):141–150. https://doi.org/10.1016/S0960-8524(99)00179-0
Hassine-Zaafrane M, Sdiri-Loulizi K, Kaplon J, Ben Salem I, Pothier P, Aouni M, Ambert-Balay K (2014) Molecular detection of human Noroviruses in influent and effluent samples from two biological sewage treatment plants in the region of Monastir, Tunisia. Food Environ Virol 6:125–131. https://doi.org/10.1007/s12560-014-9147-3
Hassine-Zaafrane M, Kaplon J, Ben Salem I, Sdiri-Loulizi K, Sakly N, Pothier P, Aouni M, Ambert-Balay K (2015) Detection and genotyping of group A rotavirus isolated from sewage samples in Monastir, Tunisia between April 2007 and April 2010. J ApplMicrobiol 119(5):1443–1453. https://doi.org/10.1111/jam.12920
He XQ, Cheng L, Zhang DY, Xie XM, Wang DH, Wang Z (2011) One-year monthly survey of rotavirus, astrovirus and norovirus in three sewage treatment plants (STPs) in Beijing, China and associated health risk assessment. Water SciTechnol 64(6):1202–1210
Ibrahim C, Cherif N Hammami S, Pothier P, Hassen A (2015) Quantification and molecular characterization of norovirus after two wastewater treatment procedures. Water Air Soil Pollut 226:187–193. https://doi.org/10.1007/s11270-015-2402-x
Ibrahim C, Chérif N, Hammami S, Pothier P, Hassen A (2016) Quantification and genotyping of Rotavirus A within two wastewater treatment processes. Clean Soil Air Water 44(4):393–401
Ibrahim C, Hammami S, Mejri S, Mehri I, Pothier P, Hassen A (2017a) Detection of Aichi virus genotype B in two lines of wastewater treatment processes. MicrobPathog 109:305–312. https://doi.org/10.1016/j.micpath.2017.06.001
Ibrahim C, Mehri I, Hammami S, Mejri S, Hassen A, Pierre P (2017b) Removal of human astroviruses from hospital wastewater by two biological treatment methods: natural oxidizing lagoons and rotating biodisks. Desalin Water Treat 89:287–296. https://doi.org/10.5004/dwt.2017.21356
Ibrahim C, Hassen A, Pothier P, Mejri S, Hammami S (2018) Molecular detection and genotypic characterization of enteric adenoviruses in a hospital wastewater. Environ Sci Pollut Res Int 25(11):10977–10987. https://doi.org/10.1007/s11356-018-1399-2
International Committee on Taxonomy of viruses (2016) Available from: http://www.ictvonline.org/virusTaxonomy.asp. Accessed: 2016-08.
Jayaram H, Estes MK, Prasad BV (2004) Emerging themes in rotavirus cell entry, genome organization, transcription and replication. Virus Res 101(1):67–81. https://doi.org/10.1016/j.virusres.2003.12.007
Kaas L, Gourinat AC, Urbès F, Langlet J (2016) A 1-year study on the detection of human enteric viruses in New Caledonia. Food Environ Virol 8(1):46–56. https://doi.org/10.1007/s12560-015-9224-2
Kargar M, Javdani N, Najafi A, Tahamtan Y (2013) First molecular detection of the group. A rotavirus in urban and hospital sewage systems by nested-RT PCR in Shiraz, Iran. J Environ Health SciEng 11(1):4. https://doi.org/10.1186/2052-336X-11-4
Kitajima M, Iker BC, Pepper IL, Gerba CP (2014) Relative abundance and treatment reduction of viruses during wastewater treatment processes-identification of potential viral indicators. Sci Total Environ 488–489:290–296. https://doi.org/10.1016/j.scitotenv.2014.04.087
La Rosa G, Pourshaban M, Iaconelli M, Muscillo M (2010) Quantitative real time of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy. Ann Ist Super Sanita 46(3):266–273. https://doi.org/10.4415/ANN-10-03-07
Leifels M, Hamza IA, Krieger M, Wilhelm M, Mackowiak M, Jurzik L (2016) From lab to lake—evaluation of current molecular methods for the detection of infectious enteric viruses in complex water matrices in an urban area. PLoS One 11(11):e0167105. https://doi.org/10.1371/journal.pone.0167105
Lever A, Desselberger U (2016) Rotavirus replication and the role of cellular lipid droplets: New therapeutic targets? J Formos Med Assoc 115(6):389–394. https://doi.org/10.1016/j.jfma.2016.02.004
Lewis GD, Melcaft TG (1988) Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water and sediment samples. Appl Environ Microbiol 54:1983–1988
Lizasoain A, Tort LFL, García M, Gillman L, Alberti A, Leite JPG, Miagostovich MP, Pou SA, Cagiao A, Razsap A, Huertas J, Berois M, Victoria M, Colina R (2018) Human enteric viruses in a wastewater treatment plant: evaluation of activated sludge combined with UV disinfection process reveals different removal performances for viruses with different features. Lett Appl Microbiol 66(3):215–221. https://doi.org/10.1111/lam.12839
Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo EA, Iturriza-Gómara M, Maes P, Patton JT, Rahman M, Van Ranst M (2008) Full genome-based classification of rotaviruses reveals a common origin between human Wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J Virol 82:3204e19. https://doi.org/10.1128/JVI.02257-07
Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Banyai K, Brister JR, Buesa J, Esona MD, Estes MK, Gentsch JR, Iturriza-Gómara M, Johne R, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Parreño V, Rahman M, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Patton JT, Desselberger U, Van Ranst M (2011) Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch Virol 156:1397e413. https://doi.org/10.1007/s00705-011-1006-z
Matthijnssens J, Otto PH, Ciarlet M, Desselberger U, Van Ranst M, Johne R (2012) VP6-sequence-based cut off values as a criterion for rotavirus species demarcation. Arch Virol 157:1177–1182. https://doi.org/10.1007/s00705-012-1273-3
Miagostovish MP, Ferreira FF, Guimarares FR, Fumian TM, Diniz-Mendes L, Luz SL, Silva LA, Leite JP (2008) Molecular detection and characterization of gastroenteritis viruses occurring naturally in the stream waters of Manaus, central Amazonia, Brazil. Appl Envi Microbiol 74:375–382. https://doi.org/10.1128/AEM.00944-07
Motayo BO, Adeniji AJ, Faneye AO (2016) First molecular detection and VP7 (G) genotyping of group A rotavirus by semi-nested RT-PCR from sewage in Nigeria. Rev Inst Med Trop Sao Paulo 58:74. https://doi.org/10.1590/S1678-9946201658074
Murray PR, Rosenthal KS, Pfaller MA (2016) Medical microbiology 8th edition. Elsevier http://evolve.elsevier.com/Murray/microbiology/
Navarro A, Trask SD, Patton JT (2013) Generation of genetically stable recombinant rotaviruses containing novel genome rearrangements and heterologous sequences by reverse genetics. J Virol 87(11):6211–6220. https://doi.org/10.1128/JVI.00413-13
O’Brien E, Nakyazze J, Wu H, Kiwanuka N, Cunningham W, Kaneene JB, Xagoraraki I (2017) Viral diversity and abundance in polluted waters in Kampala, Uganda. Water Res 127:41–49. https://doi.org/10.1016/j.watres.2017.09.063
Pang XL, Preiksaitis JK, Lee BE (2014) Enhanced enteric virus detection in sporadic gastroenteritis using a multi-target real-time PCR panel: a one-year study. J Med Virol. 86:1594–1601. https://doi.org/10.1002/jmv.23851
Prado T, Silva DM, Guilayn WC, Rose TL, Gaspar AM, Miagostovish MP (2011) Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Res 45(3):1287–1297. https://doi.org/10.1016/j.watres.2010.10.012
Prevost B, Lucas FS, Goncalves A, Richard F, Moulin L, Wurtzer S (2015) Large-scale survey of enteric viruses in river and waste water underlines the health status of the local population. Environ Int 79:42–50. https://doi.org/10.1016/j.envint.2015.03.004
Qiu Y, Lee BE, Neumann N, Ashbolt N, Craik S, Maal-Bared R, Pang XL (2015) Assessment of human virus removal during municipal wastewater treatment in Edmonton, Canada. J Appl Microbiol. 119:1729–1739
Rega Institute, KU Leuven, Belgium (2017) Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/7th-RCWG-meeting. accessed on 25 September 2017.
Rodriguez-Diaz J, Querales L, Caraballo L, Vizzi E, Liprandi F, Takiff H, Betancourt WQ (2009) Detection and characterization of waterborne gastroenteritis viruses in urban sewage and sewage polluted river waters in Caracas, Venezuela. App Environ Microbiol 75(2):387–394. https://doi.org/10.1128/AEM.02045-08
Ruggeri FM, Bonomo P, Ianiro G, Battistone A, Delogu R, Germinario C, Chironna M, Triassi M, Campagnuolo R, Cicala A, Giammanco GM, Castiglia P, Serra C, Gaggioli A, Fiore L (2015) Rotavirus genotypes in sewage treatment plants and in children hospitalized with acute diarrhea in Italy in 2010 and 2011. Appl Environ Microbiol 81(1):241–249. https://doi.org/10.1128/AEM.02695-14
Sano D, Amarasiri M, Hata A, Watanabe T, Katayama H (2016) Risk management of infectious viral diseases in wastewater reclamation and reuse: review. Environ Int 91:220–229. https://doi.org/10.1016/j.envint.2016.03.001
Sdiri-Loulizi K, Gharbi-Khélifi H, de Rougemont A, Chouchane S, Sakly N, Ambert-Balay K, Hassine M, Guédiche MN, Aouni M, Pothier P (2008) Acute infantile gastroenteritis associated with human enteric viruses in Tunisia. J ClinMicrobiol 46(4):1349–1355. https://doi.org/10.1128/JCM.02438-07
Sdiri-Loulizi K, Hassine M, Aouni Z, Gharbi-Khelifi H, Chouchane S, Sakly N, Neji-Guédiche M, Pothier P, Aouni M, Ambert-Balay K (2010) Detection and molecular characterization of enteric viruses in environmental samples in Monastir, Tunisia between January 2003 and April 2007. J Appl Microbiol 109(3):1093–1104. https://doi.org/10.1111/j.1365-2672.2010.04772.x
Staggemeier R, Heck TM, Demoliner M, Ritzel RG, Röhnelt NM, Girardi V, Venker CA, Spilki FR (2017) Enteric viruses and adenovirus diversity in waters from 2016 Olympic venues. Sci Total Environ 586:304–312. https://doi.org/10.1016/j.scitotenv.2017.01.223
Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization—Coordinated Global Rotavirus Surveillance Network (2016) Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 62(Suppl 2):S96–S105. https://doi.org/10.1093/cid/civ1013
Tonani KA, Padula JA, Julião FC, Fregonesi BM, Alves RI, Sampaio CF, Beda CF, Hachich EM, Segura-Muñoz SI (2013) Persistence of giardia, cryptosporidium, rotavirus, and adenovirus in treated sewage in São Paulo state, Brazil. J Parasitol 99(6):1144–1447. https://doi.org/10.1645/12-121.1
Trask SD, Taraporewala ZF, Boehme KW, Dermody TS, Patton JT (2010) Dual selection mechanisms drive efficient single-gene reverse genetics for rotavirus. Proc Natl Acad Sci USA 107(43):18652–18657. https://doi.org/10.1073/pnas.1011948107
Turki Y, Mehri I, Lajnef R, Rejab A, Ben Khessairi A, Cherif H, Ouzari H, Hassen A (2017) Biofilms in bioremediation and wastewater treatment: characterization of bacterial community structure and diversity during seasons in municipal wastewater treatment process. Environ Sci Pollut Res Int 24(4):3519–3530. https://doi.org/10.1007/s11356-016-8090-2
Varela MF, Monteiro S, Rivadulla E, Santos R, Romalde JL (2018) Development of a novel digital RT-PCR method for detection of human sapovirus in different matrices. J Virol Methods 254:21–24. https://doi.org/10.1016/j.jviromet.2018.01.005
Zhou N, Lv D, Wang S, Lin X, Bi Z, Wang H, Wang P, Zhang H, Tao Z, Hou P, Song Y, Xu A (2016) Continuous detection and genetic diversity of human rotavirus A in sewage in eastern China, 2013–2014. Virol J 13(1):153. https://doi.org/10.1186/s12985-016-0609-0
Acknowledgments
This study was supported by the Centre of Research and Water Technologies (CERTE) (Techno Park of Borj-Cédria, Tunisia) as part of a project program contract 2015–2018 and a close collaboration between LTVRH and the National Reference Centre of Enteric Viruses (Dijon, France).
Conflict of interest
The authors declare that they have no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerald Thouand
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibrahim, C., Hammami, S., Pothier, P. et al. The performance of biological and tertiary wastewater treatment procedures for rotaviruses A removal. Environ Sci Pollut Res 27, 5718–5729 (2020). https://doi.org/10.1007/s11356-019-05487-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05487-2