Abstract
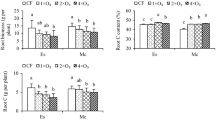
High O3 exposure affects the forest growth and soil characteristics. Although there is substantial evidence that O3 does impose a stress on forest trees, the effects of O3 on roots and soil of evergreen broad-leaved tree species in South China remain unknown. The effects of ozone (O3) fumigation on the root biomass, root morphology, root nutrient, soil physical, and chemical properties were examined in Cinnamomum camphora seedlings grown under four O3 treatments (charcoal-filtered air (CF) or O3 at 1×, 2× and 4× ambient concentration). O3 significantly decreased root biomass and root carbon (C). Regardless of O3 level, elevated O3 significantly resulted in reduced root surface area, volume, number of forks, and specific root length (SRL). The percentages of fine to total root in terms of root surface area and root volume of seedlings under the CF and 1 × O3 treatments were significantly higher than those of seedlings under the 4 × O3 treatment, indicating that high O3 level impaired the growth performance of fine roots. O3 affected root growth and structures, which increased soil bulk density and reduced soil total porosity and void ratio. The soil pH under all O3 fumigation treatments significantly increased compared with CF treatment, whereas the organic matter significantly decreased. In conclusion, although the increased O3 level enhanced root N and P under 2 and 4 × O3 treatments compared with 1 × O3 treatment as compensation mechanisms to prevent O3-induced decrease in root C gain and root functions, O3 still decreased the root biomass and root tips, and changed the soil physical and chemical properties.



Similar content being viewed by others
References
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C (1974) Chemical analysis of ecological materials. Blackwell Scientific Publications, Oxford
Andersen CP (2003) Source-sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol 157:213–228
Anderson LJ, Comas LH, Lakso AN, Eissenstat DM (2003) Multiple risk factors in root survivorship: a 4-year study in Concord grape. New Phytol 158:489–501
Aneja MK, Sharma S, Fleischmann F, Stich S, Heller W, Bahnweg G, Munch JC, Schloter M (2007) Influence of ozone on litter quality and its subsequent effects on the initial structure of colonizing microbial communities. Microb Ecol 54:151–160
Bai WM, Zhou M, Fang Y, Zhang WH (2015) Differences in spatial and temporal root lifespan of temperate steppes across Inner Mongolia grasslands. Biogeosci Discuss 12(23):19999–20023
Birouste M, Zamora-Ledezma E, Bossard C, Pérez-Ramos IM, Roumet C (2014) Measurement of fine root tissue density: a comparison of three methods reveals the potential of root dry matter content. Plant Soil 374(1–2):299–313
Blake GR, Hartge KH (1986) Bulk density. In: Klute A (ed) Methods of soil analysis, part 1. Physical and mineralogical methods. American Society of Agronomy, Madison, pp 363–382
Braun S, Zugmaier U, Thomas V, Flückiger W (2004) Carbohydrate concentrations in different plant parts of young beech and spruce along a gradient of ozone pollution. Atmos Environ 38:2399–2407
Broberg MC, Uddling J, Mills G, Pleijel H (2017) Fertilizer efficiency in wheat is reduced by ozone pollution. Sci Total Environ s607–608:876–880
Calvo E, Martin C, Sanz MJ (2007) Ozone sensitivity differences in five tomato cultivars: visible injury and effects on biomass and fruits. Water Air Soil Pollut 186:167–181
Chappelka AH, Chevone BI (1992) Trees responses to ozone. In: Lefhon AS (ed) Surface level ozone exposure and their effects on vegetation. Lewis Publishers, Chelsea, pp 271–309
Chen Z, Wang X, Feng Z, Xiao Q, Duan X (2009) Impact of elevated O3 on soil microbial community function under wheat crop. Water Air Soil Pollut 198(1–4):189–198
Chen Z, Wang XK, Yao FF, Zheng FX, Feng ZZ (2010) Elevated ozone changed soil microbial community in a rice paddy. Soil Sci Soc Am J 74(3):829–837
Craine JM, Froehle J, Tilman DG, Wedin DA, Chapin FS (2001) The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos 93:274–285
Dai L, Li P, Shang B, Liu S, Yang A, Wang Y, Feng Z (2017) Differential responses of peach (Prunus persica) seedlings to elevated ozone are related with leaf mass per area, antioxidant enzymes activity rather than stomatal conductance. Environ Pollut 227:380–388
Derwent RG, Simmonds PG, Manning AJ, Spain TG (2007) Trends over a 20-year period from 1987 to 2007 in surface ozone at the atmospheric research station, Mace Head, Ireland. Atmos Environ 41:9091–9098
Dighton J, Jansen EE (1991) Atmospheric pollutants and ectomycorrhizae. More questions than answers? Environ Pollut 73:179–204
Dizengremel P, Le Thiec D, Hasenfratz-Sauder MP, Vaultier MN, Bagard M, Jolivet Y (2009) Metabolic-dependent changes in plant cell redox power after ozone exposure. Plant Biol 11(1):35–42
Fowler D, Amann M, Anderson R, Ashmore MR, Cox P, Depledge M, Derwent D, Grennfelt P, Hewitt N, Hov O, Jenkin M, Kelly F, Liss P, Pilling M, Pyle J, Slingo J, Stevenson D (2008) Ground-level ozone in the 21st century: future trends, impacts and policy implications. Policy Document 15/08, The Royal Society, London
Gimeno BS, Bermejo V, Sanz J, de la Torre D, Elvira S (2004) Growth response to ozone of annual species from Mediterranean pastures. Environ Pollut 132:297–306
Grantz DA (2003) Ozone impacts on cotton: towards an integrated mechanism. Environ Pollut 126:331–344
Grebenc T, Kraigher H (2007) Changes in the community of ectomycorrhizal fungi and increased fine root number under adult beech trees chronically fumigated with double ambient ozone concentration. Plant Biol 9:279–287
Guangzhou weather bureau (2015) Available at: http://data.gz121.gov.cn/weather/history_weather_fy.jsp (accessed 27.12.17)
Haberer K, Grebenc T, Alexou M, Gessler A, Kraigher H, Rennenberg H (2007) Effects of long-term free-air ozone fumigation on δ15N and total N in Fagus sylvatica and associated mycorrhizal fungi. Plant Biol 9:242–252
Hendrick RL, Pregitzer KS (1993) Patterns of fine roots mortality in two sugar maple forests. Nature (London) 361:59–61
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11(12):610–617
Hill JO, Simpson RJ, Moore AD, Chapman DF (2006) Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 286:7–19
Hoshika Y, Tatsuda S, Watanabe M, Wan XN, Watanabe Y, Saito H (2013) Effect of ambient ozone at the somma of Lake Mashu on growth and leaf gas exchange in Betula ermanii and Betula platyphylla var. japonica. Environ Exp Bot 90:12–16
Hoshika Y, Carrari E, Zhang L, Carriero G, Pignatelli S, Fasano G, Materassi A, Paoletti E (2018) Testing a ratio of photosynthesis to O3 uptake as an index for assessing O3-induced foliar visible injury in poplar trees. Environ Sci Pollut Res 25:8113–8124
Huttunen S, Manninen S (2013) A review of ozone responses in Scots pine (Pinus sylvestris). Environ Exp Bot 90:17–31
Inclán R, Gimeno BS, Dizengremel P, Sanchez M (2005) Compensation processes of Aleppo pine (Pinus halepensis Mill.) to ozone exposure and drought stress. Environ Pollut 137(3):517–524
Inclán R, Gimeno BS, Peñuelas J, Gerant D, Quejido A (2011) Carbon isotope composition, macronutrient concentrations, and carboxylating enzymes in relation to the growth of Pinus halepensis Mill. When subject to ozone stress. Water Air Soil Pollut 214(1–4):587–598
Institute of Soil Sciences, Chinese Academy of Sciences (ISSCAS) (1978) Physical and chemical analysis methods of soils (in Chinese). Shanghai Science Technology Press, Shanghai
Kasurinen A, Keinänen MM, Kaipainen S, Nilsson LO, Vapaavuori E, Kontro MH, Holopainen T (2005) Below-ground responses of silver birch trees exposed to elevated CO2 and O3 levels during three growing seasons. Glob Chang Biol 11(7):1167–1179
Keeney DR (1982) Nitrogen-availability indices. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis part 2 chemical and microbiological properties. American Society of Agronomy, Madison, pp 711–730
King JS, Kubiske ME, Pregitzer KS, Hendrey GR, McDonald EP, Giardina CP, Quinn VS, Karnosky DF (2005) Tropospheric O3 compromises net primary production in young stands of trembling aspen, paper birch and sugar maple in response to elevated atmospheric CO2. New Phytol 168: 623–636
Kohler-Milleret R, Le Bayon RC, Chenu C, Gobat JM, Boivin P (2013) Impact of two root systems, earthworms and mycorrhizae on the physical properties of an unstable silt loam Luvisol and plant production. Plant Soil 370(1–2):251–265
Kolb TE, Matyssek R (2001) Limitations and perspectives about scaling ozone impacts in trees. Environ Pollut 115:373–393
Matyssek R, Sandermann H (2003) Impact of ozone on trees: an ecophysiological perspective. In: Esser K, Lüttge U, Beyschlag W, Hellwig F (eds) An ecophysiological perspective. Progress in botany, vol 64. Springer-Verlag, Berlin Heidelberg, pp 349–404
Matyssek R, Bytnerowicz A, Karlsson PE, Paoletti E, Sanz M, Schaub M, Wieser G (2007) Promoting the O3 flux concept for European forest trees. Environ Pollut 146:587–607
Matyssek R, Karnosky DF, Wieser G, Percy K, Oksanen E, Grams TEE, Kubiske M, Hanke D, Pretzsch H (2010) Advances in understanding ozone impact on forest trees: messages from novel phytotron and free-air fumigation studies. Environ Pollut 158:1990–2006
Milleret R, Le Bayon C, Lamy F, Gobat JM, Boivin P (2009) Impact of root, mycorrhiza and earthworm on soil physical properties as assessed by shrinkage analysis. J Hydrol 373:499–507
Nikolova PS, Andersen CP, Blaschke H, Matyssek R, Häberle KH (2010) Belowground effects of enhanced tropospheric ozone and drought in a beech/ spruce forest (Fagus sylvatica L./Picea abies [L.] Karst). Environ Pollut 158:1071–1078 Spec. Issue
Pääkkönen E, Holopainen T (1995) Influence of nitrogen supply on the response of clones of birch (Betula pendula Roth.) to ozone. New Phytol 129(4):595–603
Pääkkönen E, Vahala J, Pohjola M, Holopainen T, Kärenlampi L (1998) Physiological, stomatal and ultrastructural ozone responses in birch (Betula pendula Roth) are modified by water stress. Plant Cell Environ 21:671–684
Pell EJ, Landry LG, Eckardt NA, Glick RE (1994) Air pollution and rubisco: effects and implications. In: Alscher RG, Wellburn AR (eds) Plant responses to the gaseous environment. Chapman & Hall, London, pp 239–253
Pleijel H, Eriksen AB, Danielsson H, Bondesson N, Selldén G (2006) Differential ozone sensitivity in an old and a modern Swedish wheat cultivar-grain yield and quality, leaf chlorophyll and stomatal conductance. Environ Exp Bot 56(1):63–71
PRDAIR (2016) Guangdong-Hong Kong-Macao Pearl River Delta regional air quality monitoring network: a report of monitoring results in 2015, Report Number: PRDAIR-2015-5. Available at http://www.epd.gov.hk/epd/sites/default/files/epd/english/resources_pub/publications/files/PRD_2015_report_en-1.pdf
Rasheed MU, Kasurinen A, Kivimäenpää M, Ghimire R, Häikiö E, Mpamah P, Holopainen JK, Holopainen T (2017) The responses of shoot-root-rhizosphere continuum to simultaneous fertilizer addition, warming, ozone and herbivory in young Scots pine seedlings in a high latitude field experiment. Soil Biol Biochem 114:279–294
Rathnayake AP, Kadono H, Toyooka S, Miwa M (2007) Statistical interferometric investigation of nano-scale root growth: effects of short-term ozone exposure on ectomycorrhizal pine (Pinus densiflora) seedlings. J For Res 12:393–402
Ribas A, Peñuelas J, Elvira S, Gimeno BS (2005) Ozone exposure induces the activation of leaf senescence related processes and morphological and growth changes in seedlings of Mediterranean tree species. Environ Pollut 134:291–300
Samuelson LJ, Kelly JM, Mays PA, Edwards GS (1996) Growth and nutrition of Quercus rubra L. seedlings and mature trees after three seasons of ozone exposure. Environ Pollut 91:317–323
Sanz J, Muntifering RB, Bermejo V, Gimeno BS, Elvira S (2005) Ozone and increased nitrogen supply effects on the yield and nutritive quality of Trifolium subterraneum. Atmos Environ 39:5899–5907
Schulte EE, Kaufmann C, Peter JB (1991) The influence of sample size and heating time on soil weight loss-on-ignition. Commun Soil Sci Plant Anal 22:159–168
Simpson D, Arneth A, Mills G, Solberg S, Uddling J (2014) Ozone — the persistent menace: interactions with the n cycle and climate change. Curr Opin Environ Sustain 9-10:9–19
Vingarzan R (2004) A review of surface ozone background levels and trends. Atmos Environ 38:3431–3442
Vollsnesa AV, Krusea OMO, Eriksenb AB, Oxaala U, Futsaethera CM (2010) In vivo root growth dynamics of ozone exposed Trifolium subterraneum. Environ Exp Bot 69:183–188
Wang YL, Tang JW, Zhang HL, Gao ZQ, Kou TJ (2014) Aggregate-associated organic carbon and nitrogen impacted by the long-term combined application of rice straw and pig manure in red soils in South China. Soil Sci 179:522–528
Weigt RB, Häberle KH, Millard P, Metzger U, Ritter W, Blaschke H, Göttlein A, Matyssek R (2012) Ground-level ozone differentially affects nitrogen acquisition and allocation in mature European beech (Fagus sylvatica) and Norway spruce (Picea abies) trees. Tree Physiol 32(10):1259–1273
Weng Q, Yang S (2004) Managing the adverse thermal effects of urban development in a densely populated Chinese city. J Environ Manag 70(2):145–156
Yamaji K, Julkunen-Tiitto R, Rousi M, Freiwald V, Oksanen E (2003) Ozone exposure over two growing seasons alters root-to-shoot ratio and chemical composition of birch (Betula pendula Roth). Glob Chang Biol 9:1363–1377
Yoshida LC, Gamon JA, Andersen CP (2001) Differences in above- and below-ground responses to ozone between two populations of a perennial grass. Plant Soil 233:203–211
Zak DR, Pregitzer KS, King JS, Holmes WE (2000) Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis. New Phytol 147:201–222
Zhang B, Zhang TL, Zhao QG (1996) Soil erosion in various farming systems in subtropical China. Pedosphere 6:225–234
Zheng Y, Shimizu H, Barnes JD (2002) Limitations to CO2 assimilation in ozone-exposed leaves of Plantago major. New Phytol 155:67–78
Zouzoulas D, Koutroubas SD, Vassiliou G, Vardavakis E (2009) Effects of ozone fumigation on cotton (Gossypium hirsutum L.) morphology, anatomy, physiology, yield and qualitative characteristics of fibers. Environ Exp Bot 67:293–303
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pan, L., Lie, Gw., Xue, L. et al. Changes of Cinnamomum camphora root characteristics and soil properties under ozone stress in South China. Environ Sci Pollut Res 26, 30684–30692 (2019). https://doi.org/10.1007/s11356-019-05199-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05199-7