Abstract
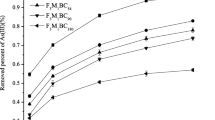
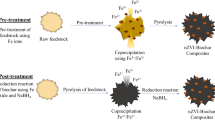
In this study, a novel Fe–Mn–Ce oxide-modified biochar composite (FMCBC) was synthesized via pyrolysis to enhance the adsorption capacity of biochar (BC). Scanning electron microscopy-energy-dispersive X-ray spectroscopy confirmed that Fe, Mn, and Ce were successfully loaded onto the surface of the BC. A series of adsorption experiments showed that the FMCBC exhibited improved adsorption of As(III) in an aqueous environment. The adsorption process was well expressed by the pseudo-second-order kinetic model. The adsorption capacity of FMCBC reached 8.74 mg L−1, which was 3.27 times greater than that of BC. The pH of the solution significantly influenced the adsorption of As(III), where the amount of As(III) adsorbed by FMCBC was maximized at pH 3. A high phosphate concentration inhibited adsorption, whereas nitrate and sulfate ions promoted As(III) adsorption and increased the FMCBC adsorption capacity. Similarly, with increasing humic acid concentration, the adsorption capacity of FMCBC for As(III) decreased; however, a low concentration of humic acid promoted adsorption. X-ray photoelectron spectroscopy analysis revealed that the adsorption of As(III) by FMCBC occurred through redox and surface complexation reactions. Therefore, FMCBC has excellent potential for purifying arsenic-contaminated water.





Similar content being viewed by others
References
Baig SA, Sheng TT, Sun C, Xue XQ, Tan LS, Xu XH (2014) Arsenic removal from aqueous solutions using Fe3O4-HBC composite effect of calcination on adsorbents performance. PLoS One 4:3979–3986
Bakshi S, Banik C, Rathke SJ, Laird DA (2018) Arsenic sorption on zero-valent iron-biochar complexes. Water Res 137:153–163
Bolan N, Mahimairaja S, Kunhikrishnan A, Choppala G (2013) Phosphorus–arsenic interactions in variable-charge soils in relation to arsenic mobility and bioavailability. Sci Total Environ 463–464:1154–1162
Chaudhry SA, Khan TA, Ali I (2017) Equilibrium, kinetic and thermodynamic studies of Cr(VI) adsorption from aqueous solution onto manganese oxide coated sand grain (MOCSG). J Mol Liq 236:320–330
Chen RZ, Zhang ZY, Lei ZF, Sugiura F (2012) Preparation of iron-impregnated tablet ceramic adsorbent for arsenate removal from aqueous solutions. Desalination 286:56–62
Chen B, Zhu ZL, Ma J, Qiu YL, Chen JH (2013) Surfactant assisted Ce–Fe mixed oxide decorated multiwalled carbon nanotubes and their arsenic adsorption performance. J Mater Chem A 1:11355–11367
Chowdhury SR, Yanful EK (2010) Arsenic and chromium removal by mixed magnetite–maghemite nanoparticles and the effect of phosphate on removal. J Environ Manag 91(11):2238–2247
Coughlin RW, Ezra FS (2018) Role of surface acidity in the adsorption of organic pollutants on the surface of carbon. Environ Sci Technol 2(4):291–297
Ding H, Zhao YL, Duan QL, Wang JW, Zhang K, Ding GY, Xie XM, Ding CM (2017) Efficient removal of phosphate from aqueous solution using novel magnetic nanocomposites with Fe3O4@SiO2 core and mesoporous CeO2 shell. J Rare Earths 35(10):984–994
Dixit S, Hering JG (2003) Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Technol 37(18):4182–4189
Dou XM, Zhang Y, Zhao B, Wu XM, Wu ZY, Yang M (2011) Arsenate adsorption on an Fe–Ce bimetal oxide adsorbent: EXAFS study and surface complexation modeling. Colloids Surf A Physicochem Eng Asp 379(1–3):109–115
Ghaneian MT, Bhatnagar A, Ehrampoush MH, Amrollahi M, Jamshidi B, Dehvari M, Taghavi M (2017) Biosorption of hexavalent chromium from aqueous solution onto pomegranate seeds: kinetic modeling studies. Int J Environ Sci Technol 14(2):331–340
Giles DE, Mohapatra M, Issa TB, Anand S, Singh P (2011) Iron and aluminium based adsorption strategies for removing arsenic from water. J Environ Manag 92:3011–3022
Gupta K, Bhattacharya S, Nandi D, Dhar A, Maity A, Mukhopadhyay A, Chattopadhyay DJ, Ray NR, Sen P, Ghosh UC (2012) Arsenic(III) sorption on nanostructured cerium incorporated manganese oxide (NCMO): a physical insight into the mechanistic pathway. J Colloid Interface Sci 377:269–276
He JS, Matsuura KS, Chen JP (2014) A novel Zr-based nanoparticle-embedded PSF blend hollow fiber membrane for treatment of arsenate contaminated water: material development, adsorption and filtration studies, and characterization. J Membr Sci 452(15):433–445
Hsu JC, Lin CJ, Liao CH, Chen ST (2008) Removal of As(V) and As(III) by reclaimed iron-oxide coated sands. J Hazard Mater 153(1–2):817–826
Kabir F, Chowdhury S (2017) Arsenic removal methods for drinking water in the developing countries: technological developments and research needs. Environ Sci Pollut Res 24:24102–24120
Katsoyiannis IA, Zouboulis AI (2002) Removal of arsenic from contaminated water sources by sorption onto iron-oxide-coated polymeric materials. Water Res 36:5141–5155
Lafferty BJ, Ginder-Vogel M, Sparks DL (2011) Arsenite oxidation by a poorly-crystalline manganese oxide. 3. Arsenic and manganese desorption. Environ Sci Technol 45(21):9218–9223
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38(1916):2221–2295
Lee D-J, Cheng Y-L, Wong R-J, Wang X-D (2018) Adsorption removal of natural organic matters in waters using biochar. Bioresour Technol 260:413–416
Lenoble V, Laclautre C, Serpaud B, Serpaud B, Deluchat V, Bollinger JC (2003) As(V) retention and As(III) simultaneous oxidation and removal on a MnO2-loaded polystyrene resin. Sci Total Environ 326(1–3):197–207
Li T, Zhu Z, Wang DS, Yao CH, Tang HX (2006) Characterization of floc size, strength and structure under various coagulation mechanisms. Powder Technol 168(8):104–110
Li Y, Cai XJ, Guo JW, Zhou SM, Na P (2015) Fe/Ti co-pillared clay for enhanced arsenite removal and photo oxidation under UV irradiation. Appl Surf Sci 324:179–187
Lin LN, Qiu WW, Wang D, Huang Q, Song ZG, Chau HW (2017) Arsenic removal in aqueous solution by a novel Fe-Mn modified biochar composite: characterization and mechanism. Ecotoxicol Environ Saf 144:514–521
Lorenc-Grabowska E, Gryglewicz G (2005) Adsorption of lignite-derived humic acids on coal-based mesoporous activated carbons. J Colloid Interface Sci 284(2):416–423
Malik AH, Khan ZM, Mahmood Q, Nasreen S, Bhatti ZA (2009) Perspectives of low cost arsenic remediation of drinking water in Pakistan and other countries. J Hazard Mater 168(1):1–12
Marziyeh SM (2016) Cadmium removal from aqueous solutions using saxaul tree ash. Iran J Chem Chem Eng 35(3):45–52
Mishra AK, Ramaprabhu S (2011) Functionalized graphene sheets for arsenic removal and desalination of sea water. Desalination 282:39–45
Mohan D, Pittman J, C U, Bricka M, Smith F, Yancey B, Mohammad J, Steele PH, Alexandre-Franco MF, Gómez-Serrano V, Gong H (2007) Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci 310(1):57–73
Mohan D, Sarswat A, OK YS, Charles U, Pittman J (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—a critical review. Bioresour Technol 160:191–202
Payne KB, Abdel-Fattah TM (2005) Adsorption of arsenate and arsenite by iron-treated activated carbon and zeolites: effects of pH, temperature, and ionic strength. J Environ Sci Health 40(4):723–749
Prum C, Dolphen R, Thiravetyan P (2018) Enhancing arsenic removal from arsenic-contaminated water by Echinodorus cordifolius-endophytic Arthrobacter creatinolyticus interactions. J Environ Manag 213:11–19
Qian W, Zhao AZ, Xu RK (2013) Sorption of As(V) by aluminum-modified cropstraw-derived biochars. Water Air Soil Pollut 224:1610
Siddiqui SI, Chaudhry SA (2018) A review on graphene oxide and its composites preparation and their use for the removal of As3+ and As5+ from water under the effect of various parameters: application of isotherm, kinetic and thermodynamics. Process Saf Environ Prot 119:138–163
Vadahanambi S, Lee SH, Kim WJ, Oh IK (2013) Arsenic removal from contaminated water using three-dimensional graphene-carbon nanotube-iron oxide nanostructures. Environ Sci Technol 47(18):10510–10517
Van VN, Zafar M, Behera S, Park HS (2015) Arsenic(III) removal from aqueous solution by raw and zinc-loaded pine cone biochar: equilibrium, kinetics, and thermodynamics studies. Int J Environ Sci Technol 12(4):1283–1294
Wang CB, Liu HM, Zhang Y, Zou C, Anthony EJ (2018) Review of arsenic behavior during coal combustion: volatilization, transformation, emission and removal technologies. Prog Energy Combust Sci 68:1–28
Yin DX, Wang X, Peng B, Tan CY, Ma LQ (2017) Effect of biochar and Fe-biochar on Cd and As mobility and transfer in soil-rice system. Chemosphere 186:928–937
Yoon K, Cho DW, Tsang DCW, Bolan BN, Rinklebe J, Song H (2017) Fabrication of engineered biochar from paper mill sludge and its application into removal of arsenic and cadmium in acidic water. Bioresour Technol 246:69–75
Yu ZH, Zhou L, Huang YF, Song ZG, Qiu WW (2015) Effects of a manganese oxide-modified biochar composite on adsorption of arsenic in red soil. J Environ Manag 163:155–162
Yu Y, Zhang CY, Yang LM, Chen JP (2017) Cerium oxide modified activated carbon as an efficient and effective adsorbent for rapid uptake of arsenate and arsenite: material development and study of performance and mechanisms. Chem Eng J 315:630–638
Zhang Y, Yang M, Dou XM, He H, Wang DS (2005) Arsenate adsorption on an Fe-Ce bimetal oxide adsorbent: role of surface properties. Environ Sci Technol 39(18):7246–7253
Zhang Y, Dou XM, Zhao B, Yang M, Takayama T, Kato S (2010a) Removal of arsenic by a granular Fe–Ce oxide adsorbent: fabrication conditions and performance. Chem Eng J 162(1):164–170
Zhang K, Dwivedi V, Chi CY, Wu JS (2010b) Graphene oxide/ferric hydroxide composites for efficient arsenate removal from drinking water. J Hazard Mater 182(1–3):162–168
Zhang SX, Niu HY, Cai YQ, Zhao XL, Shi YL (2010c) Arsenite and arsenate adsorption on coprecipitated bimetal oxide magnetic nanomaterials: MnFe2O4 and CoFe2O4. Chem Eng J 158(3):599–607
Zhang LF, Zhu TY, Liu X, Zhang WQ (2016) Simultaneous oxidation and adsorption of As(III) from water by cerium modified chitosan ultrafine nanobiosorbent. J Hazard Mater 308:1–10
Zhang CN, Kibriya MG, Jasmine F, Roy S, Gao JJ, Sabarinathan M, Shinkle J, Delgado D, Ahmed A, Islam T, Eunus M, Islam MT, Hasan R, Graziano JH, Ahsan H, Pierce BL (2018) A study of telomere length, arsenic exposure, and arsenic toxicity in a Bangladeshi cohort. Environ Res 164:346–355
Zhu J, Lou Z, Wang ZX, Xu XH (2014) Preparation of iron and manganese oxides/carbon composite materials for arsenic removal from aqueous solution. Prog Chem 26(9):1551–1561
Funding
This work was supported by the National Science Foundation of China (grant number 41771525).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they no conflict of interest.
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Gao, M., Qiu, W. et al. Fe–Mn–Ce oxide-modified biochar composites as efficient adsorbents for removing As(III) from water: adsorption performance and mechanisms. Environ Sci Pollut Res 26, 17373–17382 (2019). https://doi.org/10.1007/s11356-019-04914-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04914-8