Abstract
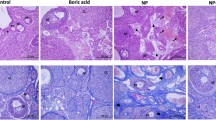
Bisphenol A (BPA) is a key endocrine-disrupting chemical (EDC) in the manufacturing industry. It is found in the structure of compounds such as polycarbonate and epoxy in combination with other chemicals. Our objective was to investigate the effect of BPA on rat ovaries. A total of 32 female rats were divided into four equal groups: In group 1 (control), vehicle was administered; in group 2, BPA 50 μg/day was administered intraperitoneally; in group 3, BPA 100 mg/kg/day was administered intraperitoneally; and in group 4, BPA 100 mg/kg/day and vitamin C (50 mg/kg) were administered intraperitoneally, while vitamin E (50 mg/kg) was administered intramuscularly. Thirty days after the treatment, the effects of BPA on the ovaries were evaluated by terminal deoxynucleotidyltransferase [TdT]-mediated dUTP-biotin nick end labeling (TUNEL) assay. There was no difference in the number of apoptotic cells between group 2 and group 4. In addition, there was no significant difference between control group and group 2, 4. However, the number of apoptotic cells per unit area was significantly increased in group 3 compared with all groups (p < 0.01, p < 0.05). In conclusion, this study showed that high doses of BPA (100 mg/kg/day) have a toxic effect on the ovaries. The fact that the number of apoptotic cells in the group administered with high dose of BPA + 50 mg/kg/day vitamin C + 50 mg/kg/day vitamin E was lower than that of the high-dose BPA-administered group shows that these vitamins may have a protective effect.

Similar content being viewed by others
References
Adewale HB, Jefferson WN, Newbold RR, Patisaul HB (2009) Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod 81:690–99
Aikawa H, Koyama S, Matsuda M, Nakahashi K, Akazome Y, Mori T (2004) Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res 315:119–124
Aydogan M, Korkmaz A, Barlas N et al (2010) Pro-oxidant effect of vitamin C coadministration with bisphenol A, nonylphenol, and octylphenol on the reproductive tract of male rats. Drug Chem Toxicol 33:193–203
Bansal AK, Bansal M, Soni G, Bhatnagar D (2005) Protective role of vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact 156:101–111
Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N (1995) Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect 103:608–612
Carney EW, Hoberman AM, Farmer DR, Kapp RW Jr, Nikiforov AI, Bernstein M, Hurtt ME, Breslin WJ, Cagen SZ, Daston GP (1997) Estrogen modulation: tiered testing for human hazard evaluation. American industrial health council, reproductive and developmental effects subcommittee. Reprod Toxicol 11:879–892
Ginsberg G, Rice DC (2009) Does rapid metabolism ensure negligible risk from bisphenol A? Environ Health Perspect 117:1639–1643
Grasselli F, Baratta L, Baioni L, Bussolati S, Ramoni R, Grolli S, Basini G (2010) Bisphenol A disrupts granulosa cell function. Domest Anim Endocrinol 39:34–39
Iida H, Maehara K, Doiguchi M, Mōri T, Yamada F (2003) Bisphenol A-induced apoptosis of cultured rat Sertoli cells. Reprod Toxicol 17:457–464
Korkmaz A, Ahbab MA, Kolankaya D, Barlas N (2010) Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem Toxicol 48:2865–2871
Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S (2003) Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res 45:345–356
Laws SC, Carey SA, Ferrell JM, Bodman GJ, Cooper RL (2000) Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol Sci 54:154–167
Lee S, Suk K, Kim IK, Jang IS, Park JW, Johnson VJ, Kwon TK, Choi BJ, Kim SH (2008) Signaling pathways of bisphenol A-induced apoptosis in hippocampal neuronal cells: role of calcium-induced reactive oxygen species, mitogen-activated protein kinases, and nuclear factor-kappa B. J Neurosci Res 86:2932–2942
Maffini MV, Rubin BS, Sonnenschein C, Soto AM (2006) Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol 254-255:179–186
Munoz-de-Toro M, Markey CM, Wadia PR et al (2005) Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology 146:4138–4147
Olea N, Pulgar R, Perez P et al (1996) Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect 104:298–305
Patel S, Brehm E, Gao L, Rattan S, Ziv-Gal A, Flaws JA (2017) Bisphenol A exposure, ovarian follicle numbers, and female sex steroid hormone levels: results from a CLARITY-BPA study. Endocrinology 158:1727–1738
Prins GS, Patisaul HB, Belcher SM, Vandenberg LN (2018) CLARITY-BPA academic laboratory studies identify consistent low-dose bisphenol A effects on multiple organ systems. Basic Clin Pharmacol Toxicol. https://doi.org/10.1111/bcpt13125
Rodriguez HA, Santambrosio N, Santamaria CG et al (2010) Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol 30:550–557
Tasdemir N, Kilic S, Lortlar N, Yuksel B, Goker U, Ozaksit G (2012) Time dependent influence of etonogestrel on the caspase-3 immuno reactivity and apoptotic indexes of rat uterus and ovaries. Gynecol Endocrinol 28:463–467
Toyama Y, Yuasa S (2004) Effects of neonatal administration of 17 beta-estradiol, beta-estradiol 3-benzoate, or bisphenol A on mouse and rat spermatogenesis. Reprod Toxicol 19:181–188
Tuncdemir M, Ozturk M (2008) The effects of ACE inhibitor and angiotensin receptor blocker on clusterin and apoptosis in the kidney tissue of streptozotocin-diabetic rats. J Mol Histol 39:605–616
Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM (2009) Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30:75–95
vom Saal FS, Akingbemi BT, Belcher SM et al (2007) Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol 24:131–138
Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM (2007) In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol 24:178–198
Xu J, Osuga Y, Yano T, Morita Y, Tang X, Fujiwara T, Takai Y, Matsumi H, Koga K, Taketani Y, Tsutsumi O (2002) Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochem Biophys Res Commun 292:456–462
Zoeller RT, Bansal R, Parris C (2005) Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 146:607–612
Funding
This study was supported by the Istanbul Training and Research Hospital Research Funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics committee approval was obtained from Istanbul University, Animal Research Local Ethics Committee (date: 25.11.2010, number: 176).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bilgi, A., Abalı, R., Bilgi, P.T. et al. The apoptotic effects of bisphenol A exposure on the rat ovary: an experimental study. Environ Sci Pollut Res 26, 10198–10203 (2019). https://doi.org/10.1007/s11356-019-04487-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04487-6