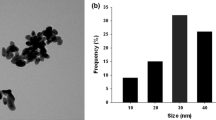
Abstract
The rapid development of nanotechnology raises questions assessment of their impact on living objects. In the present study, we evaluated the impact of nanoparticle (NP) CuO at concentrations ranging from 0.8 to 63.5 g/l in the test on wheat seedlings Triticum vulgare during 1–72 h. In the viability test (WST-test), cells were isolated from the roots of seedlings T. vulgare, 12 h not observed increase reductase activity after 24 h decreased rate of not more than 19% compared with the control. The number of dead cells in seedlings of T. vulgaris after exposure with CuO nanoparticles to the test with Evans blue increased by 5–15% compared to control. We observed that a significant increase in copper revenues leaves 4.5–8.9 times more in relation to the control and the roots—in 5–9.7 times. During the determined amount of active oxygen species, a significant proportional increase in the total pool of reactive oxygen species (ROS) in roots increased to 27.6% after exposure to NP CuO compared with the control. It is shown that in the introduction in medium, the NP CuO in the doses ranging from 3.2 to 63.5 g/l leads to DNA fragmentation and increases the fragments less than 3000 bp on 51.4–62.8%. The totality of our results influences nanoforms of copper oxide on the amount of ROS, and the viability of the genomic component of the cells shows different mechanisms of damage in the activation of a metabolic reaction, to determine the concentration of nano-CuO.











Similar content being viewed by others
References
Albanese A, Tang PS, Chan WC (2012) The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 14:1–16
Anastassopoulou J (2003) Metal–DNA interactions. J Mol Struct 651:19–26
Atha DH et al (2012) Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol 46(3):1819–1827
Barbusiński K (2009) The full-scale treatment plant for decolourisation of dye wastewater. ACEE 2(2):89–94
Bhattacharya K, Davoren M, Boertz J, Schins RP, Hoffmann E, Dopp E (2009) Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Part Fibre Toxicol 6:17
Berg MA, Coleman RS, Murphy CJ (2008) Nanoscale structure and dynamics of DNA. Phys Chem Chem Phys 10(9):1229–1242
Bonini MG, Rota C, Tomasi A, Mason RP (2006) The oxidation of 2′, 7′-dichlorofluorescin to reactive oxygen species: a self-fulfilling prophesy? Free Radic Biol Med 40(6):968–975
Carpita NC (1982) Limiting diameters of pores and the surface structure of plant cell walls. Science 218(4574):813–814
Castro-Concha LA, Escobedo RM, Miranda-Ham ML (2006) Measurement of cell viability in in vitro cultures. Methods in Mol Bio 318:71–76
Chandra R, Bharagava RN, Yadav S, Mohan D (2009) Accumulation and distribution of toxic metals in wheat (Triticum aestivum L.) and Indian mustard (Brassica campestris L.) irrigated with distillery and tannery effluents. J Hazard Mater 162(2–3):1514–1521. doi:10.1016/j.jhazmat.2008.06.040
Deryabin DG et al (2015) A zeta potential value determines the aggregate’s size of penta-substituted [60] fullerene derivatives in aqueous suspension whereas positive charge is required for toxicity against bacterial cells. J Nanobiotechnology. doi:10.1186/s12951-015-0112-6
Deryabina TD (2015) Adaptive response and tolerance limits of Triticum aestivum and Allium cepa L. to nanoparticles of copper and iron. Abstract. dissertation, Orenburg State Pedagogical University
Dimkpa CO et al (2012) CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res 14(9):1–15
Donald AH, Huanhua W, Elijah JP, Leveland D, Holbrook RD, Jaruga P, Dizdaroglu M, Xing B, Nelson BC (2014) Correction to copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol 48(20):12473
Dougherty GM, Rose KA, Tok JB-H, Pannu SS, Chuang FYS, Sha MY, Chakarova G, Penn SG (2008) The zeta potential of surface-functionalized metallic nanorod particles in aqueous solution. Electrophoresis 29(5):1131–1139
El-Temsah YS, Joner EJ (2010) Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol 27(1):42–49. doi:10.1002/tox.20610
Faisal M, Saquib Q, Alatar AA, Al-Khedhairy AA, Hegazy AK, Musarrat J (2013) Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J Hazard Mater 250:318–332
Feldblyum V (2013) «Nano» at the intersection of science: nano-objects, nanotechnology, nanofuture [electronic resource] Digital Library of Northern (Arctic) Federal University. MV Lomonosov. URL:http://narfu.ru/university/library/books/0706.pdf
Ganea GM, Kolic PE, El-Zahab B, Warner IM (2011) Ratiometric Coumarin—Neutral Red (CONER) Nanoprobe for Detection of Hydroxyl Radicals. Anal Chem 83(7):2576–2581
Gerald LN, Jamie RM (2006) Fluorescence detection of hydroxyl radicals. Radiat Phys Chem 75:473–478
Geremias R, Fattorini D, Favere VTD, Pedrosa RC (2010) Bioaccumulation and toxic effects of copper in common onion Allium cepa L. Chem Ecol 26(1):19–26. doi:10.1080/02757540903468144
Godymchuk AY, Savelyev GG, Zykov AP (2012) Ecology of nanomaterials. Tutorial. Moscow, Binom. Knowledge Laboratory
Gomes A, Fernandes E, Lima JL (2005) Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65:45–80
Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D, Barber DS (2007) Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ Sci Technol 41:8178–8186
Hackl EV, Kurnilova SV, Blago YP (2005) DNA structure transitions induced by divalent metal ions in aqueous solutions. Int J Biol Macromol 35(2–4):175–191
Held P (2015) An introduction to reactive oxygen species measurement of ROS in cells. BioTek Instruments 6:1–22
Henle ES, Han Z, Teng N, Rai P, Luo Y, Lim S (1999) Sequence-specific DNA cleavage by Fe2+-mediated Fenton reactions has possible biological implications. J Biol Chem 274:962–971
Higuchi Y (2004) Glutathione depletion-induced chromosomal DNA fragmentation associated with apoptosis and necrosis. J Cell Mol Med 8:455–464
Horie M, Kato H, Fujita K, Endoh S, Iwahashi H (2012) In vitro evaluation of cellular response induced by manufactured nanoparticles. Chem Res Toxicol 25(3):605–619
Hotze EM, Phenrat T, Lowry GV (2010) Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J Environ Qual 39:1909–1924
Husen A, Siddiqi KS (2014) Phytosynthesis of nanoparticles: concept, controversy and application. Nanoscale Res Lett 9:229
Jakubowski W, Bartosz G (2000) 2,7-Dichlorofluorescin oxidation and reactive oxygen species: what does it measure? Cell Biol Int 24:757–760
Kasemets K, Ivask A, Dubourguier H, Kahru A (2009) Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol in Vitro 23:1116–1122
Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44:1962–1967
Kim S, Sin H, Lee S, Lee I (2013) Influence of metal oxide particles on soil enzyme activity and bioaccumulation of two plants. J Microbiol Biotechnol 23(9):1279–1286
Korotkova AM, Davydova OK (2015) Effects of synthetic alkylresorcinols to topological changes in DNA, mediated by reactive oxygen species in a system in vitro. Vestnik of OSU 6:157–164
Korotkova AM, Sizova EA, Lebedev SV, Zyazin NN (2015) Influence of NPs Ni° on the induction of oxidative damage in Triticum vulgare. Orient J Chem 31:137–145
Kosyan DB et al (2016) Toxic effect and mechanisms of nanoparticles on freshwater infusoria. Int J Geomate 11(23):2170–2176
Koukalova B, Kovarik A, Fajkus J, Siroky J (1997) Chromatin fragmentation associated with apoptotic changes in tobacco cells exposed to cold stress. FEBS Lett 414:289–292
Koul A, Gupta SP (2013) Phytomodulatory potential of lycopene from Lycopersicum esculentum against doxorubicin induced nephrotoxicity. Indian J Exp Biol 51:635–645
Kurikka SA, Ulman A, Yan X, Yang N-L, Estourns JI (1996) DNA-based method for nationally assembling nanoparticles into macroscopic materials. Nature 382:607–609
Lebedev SV, Korotkova AM, Osipova EA (2014) Influence of Fe° nanoparticles, magnetite Fe3O4 nanoparticles, and iron (II) sulfate (FeSO4) solutions on the content of photosynthetic pigments in Triticum vulgare Russian. J Plant Physiol 61(4):564–569
Lee WM, An YJ, Yoon H, Kweon HS (2008) Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environ Toxicol Chem 27:1915–1921. doi:10.1897/07-481.1
Lin BS, Diao SQ, Li CH, Fang LJ, Qiao SC, Yu M (2004) Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J For Res-CHN 15:138–140
Lu CM, Zhang CY, Wen JQ, Wu GR, Tao MX (2002) Research of the effect of nanometer materials on germinations and growth enhancement of Glycine max and its mechanism. Soybean Sci 21:168–172
Ma X, Lee JG, Deng Y, Kolmakov A (2010) Interactions between engineered nanoparticles (ENPs) and plants. Phytotoxicity, uptake and accumulation, science of the total environment 408:3053–3061
Makarov DV (2014) Forecast of development world market nanopowders. Vestnik KRAUNTS. Phys Math Sci 1(8):97–102
Manevich Y, Held KD, Biaglow JE (1997) Coumarin-3-carboxylic acid as a detector for hydroxyl radicals generated chemically and by gamma radiation. Radiat Res 148:580–591
Masarovicova E, Kralova K (2013) Metal nanoparticles and plants. Ecol Chem Eng 20(1):9–22
Metzler DM, Erdem A, Tseng YH, Huang CP (2012) Responses of algal cells to engineered nanoparticles measured as algal cell population, chlorophyll a, and lipid peroxidation: effect of particle size and type. J Nanotechnol . doi:10.1155/2012/23728412 p
Miller RJ, Bennett S, Keller AA, Pease S, Lenihan HS (2012) TiO2 nanoparticles are phototoxic to marine phytoplankton. PLoS One 7:1–7
Nair PM, Chung IM (2014) Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ Sci Pollut Res Int 21:12709–127022
Naqvi S, Samim M, Abdin MZ, Ahmed FJ, Maitra AN, Prashant CK, Dinda AK (2010) Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. International J of Nanomedicine 5:983
Peng L, Wang B, Ren P (2005) Reduction of MTT by flavonoids in the absence of cells. Colloids Surf B: Biointerfaces 45(2):108–111
Persson P, Nilsson N, Sjoherg S (1996) Structures and bonding of orthophosphate ions at the iron oxide-aqueous interface. J of Colloid interface Sci 177(1):263–275
Pokhrel LR, Dubey B (2013) Evaluation of development al responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci Total Environ 452:321–332
Pourrut B, Pohu AL, Pruvot C, Garçon G, Verdin A, Waterlot C, Bidar G, Shirali P, Douay F (2011) Assessment of fly ash-aided phytostabilisation of highly contaminated soils after an 8-year field trial part 2. Influence on plants. Sci Total Environ 409:4504–4510
Rico CM, Peralta-Videa JR, Gardea-Torresdey JL (2015) Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense system in plants Nanotechnology and plant sciences: nanoparticles and their impact on plants. New York: Springer, 2015, 1–19 р. 10.1007/978-3-319-14502-0_1
Shi J, Abid AD, Kennedy IM, Hristova KR, Silk WK (2011) To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ Pollut 159:1277–1282
Sirota T (2000) Method for determining the antioxidant activity of superoxide dismutase and chemical compounds / Sirota, T. // Patent number 2144674 (Russia)
Sizova E, Miroshnikov S, Yausheva E, Kosyan D (2015) Comparative characteristic of toxicity of nanoparticles using the test of bacterial bioluminescence. Biosciences biotechnology research Asia 12:361–368
Thul ST (2013) Nanotechnology in agroecosystem: implications on plant productivity and its soil environment. Expert Opin Environ Biol 7:1–7
Urbanski NK, Beresewicz A (2000) Generation of hydroxyl radical initiated by interaction of Fe2+ and Cu+ with dioxygen; comparison with the Fenton chemistry. Acta Biochim Pol 47(4):951–962
Wang Z, Li N, Zhao J, White JC, Qu P, Xing B (2012a) CuO nanoparticle interaction with human epithelial cells: cellular uptake, location, export, and genotoxicity. Chem Res Toxicol 25:1512–1521
Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC, Xing B (2012b) Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ Sci Technol 46(8):4434–4441
Whitby M, Quirke N (2007) Fluid flow in carbon nanotubes and nanopipes. Nat Nanotechnol 2:87–94
Wilson K, Uolker D (2015) Principles and methods of biochemistry and molecular biology. Binom, Moscow
WST-8 patent no 2.251.850 (Canada), 6.063.587 (US) 0908453 (EP), 2757348 (JP) (2012) Measuring cell viability/cytotoxicity: cell counting Kit-8 [https://www.dojindo.com/Protocol/Cell_Proliferation_Protocol_Colorimetric.pdf]
Wu N, Fu L, Su M, Aslam M, Wung KC, Dravid VP (2004) Interaction of fatty acid monolayers with cobalt nanoparticles. Nanolett 4(2):383–386
Wu SG, Huang L, Head J, Chen DR, Kong IC, Tang YJ (2012) Phytotoxicity of metal oxide nanoparticles is related to both dissolved metal ions and adsorption of particles on seed surfaces. Pet Environ Biotechnol 3(4):126
Yoon HJ, Kim CS, Lee KY, Yang SY (2010) Antioxidant activity of Rubus coreanus fruit extract: in comparison to green tea extract. Chonnam Medical J 46(3):148–155
Zhao L, Peng B, Hernandez-Viezcas JA, Rico C, Sun Y, Peralta-Videa JR et al (2012) Stress response and tolerance of Zea mays to CeO2 nanoparticles: cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano 6(11):9615–9622. doi:10.1021/nn302975u
Zuppini A, Navazio L, Mariani P (2004) Endoplasmic reticulum stress-induced programmed cell death in soybean cells. J of Cell Science 117:2591–2598
Acknowledgements
Research carried was out with the financial support of the Russian Ministry of Education as part of the base part of the state task to carry out research projects in the Orenburg State University (project no. 342).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Korotkova, A.M., Lebedev, S.V. & Gavrish, I.A. The study of mechanisms of biological activity of copper oxide nanoparticle CuO in the test for seedling roots of Triticum vulgare . Environ Sci Pollut Res 24, 10220–10233 (2017). https://doi.org/10.1007/s11356-017-8549-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8549-9