Abstract
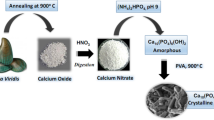
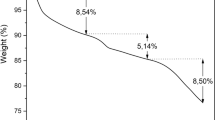
In this research, a versatile and highly efficient method for the stabilization of nanoscale zerovalent iron particles (nZVI) on the surface of ostrich bone ash (OBA) was presented as a novel inorganic adsorbent (OBA/nZVI) for the removal of Hg(II) and Pb(II) ions from aqueous solutions, even after 1 year of storage under room conditions. The removal behavior of the OBA/nZVI was assessed as a function of the initial pH, contact time, initial pollutants concentration, temperature, amount of adsorbent, effect of competitive metal ions, and ionic strength. The synthesized adsorbent was characterized by several techniques including N2 adsorption at − 196 °C, FT-IR spectroscopy, scanning electron microscopy, X-ray diffraction, and zeta potential. The results confirmed that the OBA is a good candidate as support of nZVI. The maxima adsorption capacity for Hg(II) and Pb(II) ions found from experimental results were 170 and 160 mg g−1, when the loading quantities of Fe were 20%. The equilibrium sorption data obeyed a Langmuir–Freundlich isotherm type model. The kinetic data of the adsorption followed the mechanism of the pseudo-second-order model. The thermodynamic experiments indicated that the removal of metal ions were feasible, endothermic, and spontaneous. It can be found that fresh and aged OBA/nZVI maintained its usability even after five cycles in the order: fresh (OBA/nZVI)-Hg(II) > fresh (OBA/nZVI)-Pb(II) > aged (OBA/nZVI)-Hg(II) > aged (OBA/nZVI)-Pb(II), which indicate that OBA/nZVI can be regenerated as adsorbent. The existence of Fe in the OBA/nZVI was proved by SEM-EDX results and X-ray diffraction analysis also confirmed adsorption/reduction of some of the Hg(II) to Hg0 and Pb(II) to Pb0.

















Similar content being viewed by others
References
Amiri MJ, Abedi-Koupai J, Eslamian SS, Mousavi SF, Hasheminejad H (2013) Modeling Pb(II) adsorption from aqueous solution by ostrich bone ash using adaptive neural-based fuzzy inference system. J Environ Sci Health Part A 48:543–558
Amiri MJ, Abedi-Koupai J, Eslamian SS, Arshadi M (2016) Adsorption of Pb(II) and Hg(II) ions from aqueous single metal solutions by using surfactant-modified ostrich bone waste. Desal Water Treat 57:16522–16539
Arshadi M, Soleymanzadeh M, Salvacion JWL, SalimiVahid F (2014) Nanoscale Zero-Valent Iron (NZVI) supported on sineguelas waste for Pb(II) removal from aqueous solution: Kinetics, thermodynamic and mechanism. J Colloid Interface Sci 426:241–251
Arshadi M, Faraji AR, Amiri MJ, Mehravar M, Gil A (2015) Removal of methyl orange on modified ostrich bone waste—a novel organic–inorganic biocomposite. J Colloid Interface Sci 446:11–23
Bahrololoom ME, Javidia M, Javadpoura S, Ma J (2009) Characterisation of natural hydroxyapatite extracted from bovine cortical bone ash. J Ceram Process Res 10:129–138
Bundschuh J, Farias B, Martin R, Storniolo A, Bhattacharya P, Cortes J, Bonorino G, Albouy R (2004) Groundwater arsenic in the Chaco-Pampean Plain, Argentina: case study from Robles country, Santiago del Estero Province. Appl Geochem 19:231–243
Calisir F, Roman FR, Alamo L, Perales O, Arocha MA, Akman S (2009) Removal of Cu (II) from aqueous solutions by recycled tire rubber. Desalination 249:515–518
Cason R, Lester WR (1997) Chemistry of two clay systems and three phenoxy herbicides. Proc. Okla. Acad Sci 57:116–118
Dong L, Zhua Z, Qiu Y, Zhao J (2010) Removal of lead from aqueous solution by hydroxyapatite/magnetite composite adsorbent. Chem Eng J 165:827–834
Eslamian S, Amiri MJ, Abedi-Koupai J, Karimi SS (2013) Reclamation of unconventional water using nano zero-valent iron particles: an application for groundwater. Int J Water 7:1–13
Feng Y, Gong J-L, Zenga G-M, Niua Q-Y, Zhanga H-Y, Niua C-G, Denga J-H, Yana M (2010) Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem Eng J 162:487–494
Ghasemi Z, Seif A, Ahmadi TS, Zargar B, Rashidi F, Rouzbahani GM (2012) Thermodynamic and kinetic studies for the adsorption of Hg(II) by nano-TiO2 from aqueous solution. Adv Powder Technol 23:148–156
Gotic M, Music S (2007) Mössbauer, FT-IR and FE SEM investigation of iron oxides precipitated from FeSO4 solutions. J Mol Struct 834–836:445–453
Hansen HK, Arancibia F, Gutierrez C (2010) Adsorption of copper onto agriculture waste materials. J Hazard Mater 180:442–448
Hassan SSM, Awwad NS, Aboterika AHA (2008) Removal of mercury (II) from wastewater using camel bone charcoal. J Hazard Mater 154:992–997 http://www.atsdr.cdc.gov, http://www.worldrecordacademy.com
Kadirvelu K, Kavipriya M, Karthika C, Vennilamani N, Pattabhi S (2004) Mercury (II) adsorption by activated carbon made from sago waste. Carbon 42:745–752
Kim SA, Kamala-Kannan S, Lee KJ, Park YJ, Shea PJ, Lee WH, Kim HM, Oh BT (2013) Removal of Pb(II) from aqueous solution by a zeolite-nanoscale zero-valent iron composite. Chem Eng J 217:54–60
Kizilkaya B, Tekinary AA, Dilgin Y (2010) Adsorption and removal of Cu (II) ions from aqueous solution using pretreated fish bones. Desalination 264:37–47
Li XQ, Zhang WX (2006) Iron nanoparticles: the core–shell structure and unique properties for Ni(II) sequestration. Langmuir 22:4638–4642
Liu TL, Zhao L, Sun D, Tan X (2010) Entrapment of nanoscale zero-valent iron in chitosan beads for hexavalent chromium removal from wastewater. J Hazard Mater 184:724–730
Liu T, Wang ZL, Sun Y (2015) Manipulating the morphology of nanoscale zero-valent iron on pumice for removal of heavy metals from wastewater. Chem Eng J 263:55–61
Pan X, Wang J, Zhang D (2009) Sorption of cobalt to bone char: kinetics, competitive sorption and mechanism. Desalination 249:609–614
Perrin DD, Armarego WLF, Perrin DR (1983) Purification of laboratorycChemicals. Pergamon, New York
Persson I (2010) Hydrated metal ions in aqueous solution: how regular are their structures? Pure Appl Chem 82:1901–1917
Peters F, Schwarz K, Epple M (2000) The structure of bone studied with synchrotron X-ray diffraction, X-ray absorption spectroscopy and thermal analysis. Thermochim Acta 361:131–138
Ponder SM, Darab JD, Mallouk TE (2000) Remediation of Cr(VI) and Pb(II) aqueous solutions using nanoscale zero-valent iron. Environ Sci Technol 34:2564–2569
Şeker A, Shahwan T, Eroğlu AE, Yılmaz S, Demirel Z, Dalay MC (2008) Equilibrium, thermodynamic and kinetic studies for the biosorption of aqueous lead (II), cadmium (II) and nickel (II) ions on Spirulina platensis. J Hazard Mater 154:973–980
Shi L-N, Lin Y-M, Zhang X, Chen Z-L (2011) Synthesis, characterization and kinetics of bentonite supported nZVI for the removal of Cr(VI) from aqueous solution. Chem Eng J 171:612–617
Soleymanzadeh M, Arshadi M, Salvacion JWL, SalimiVahid F (2015) A new and effective nanobiocomposite for sequestration of Cd (II) ions: Nanoscale zerovalent iron supported on sineguelas seed waste. Chem Eng Res Des 93:696–709
Sun Y, Li X, Cao XJ, Zhang W, Wang HP (2006) Characterization of zerovalent iron nanoparticles. Adv Colloid Interf Sci 120:47–56
Sun P, Liu ZT, Li ZW (2009) Particles from bird feather: a novel application of an ionic liquid and waste resource. J Hazard Mater 170:786–790
The Council of the European Communities (May 1976) Directive on pollution caused by certain dangerous substances discharged into the aquatic environment of the community [76/464/EEC]. Off J Eur Commun 129(/23):18
Tran VH, Dai Tran L, Ngoc Nguyen T (2010) Preparation of chitosan/magnetite composite beads and their application for removal of Pb(II) and Ni(II) from aqueous solution. Mater Sci Eng, C 30:304–310
Uzuma C, Shahwan T, Eroglu AE, Lieberwirth I, Scott TB, Hallam KR (2008) Application of zero-valent iron nanoparticles for the removal of aqueous Co2+ ions under various experimental conditions. Chem Eng J 144:213–220
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Wang CT, Ro SH (2007) Nanoparticle iron–titanium oxide aerogels. Mater Chem Phys 101:41–48
Zarrouk A, Hammouti B, Zarrok H, Al-Deyab SS, Messali M (2011) Temperature effect, activation energies and thermodynamic adsorption studies of L-Cysteine methyl ester hydrochloride as copper corrosion inhibitor in nitric acid 2M. Int J Electrochem Sci 6:6261–6274
Zhang H, Jin ZH, Han L, Qin CH (2006) Synthesis of nanoscale zero-valent iron supported on exfoliated graphite for removal of nitrate. Trans Nonferrous Met Soc China 16:345–349
Zhang X, Lin S, Lu XQ, Chen Z-L (2010) Removal of Pb(II) from water using synthesized kaolin supported nanoscale zero-valent iron. Chem Eng J 163:243–248
Zhang X, Lin S, Chen Z, Megharaj M, Naidu R (2011) Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: reactivity, characterization and mechanism. Water Res 45:3481–3488
Zhang J, Ding T, Zhang Z, Xu L, Zhang C (2015) Enhanced adsorption of trivalent arsenic from water by functionalized diatom silica shells. PLoS One 10(4):e0123395
Funding
The authors would like to thank Iranian Nanotechnology Initiative for supporting of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Gil, A., Amiri, M.J., Abedi-Koupai, J. et al. Adsorption/reduction of Hg(II) and Pb(II) from aqueous solutions by using bone ash/nZVI composite: effects of aging time, Fe loading quantity and co-existing ions. Environ Sci Pollut Res 25, 2814–2829 (2018). https://doi.org/10.1007/s11356-017-0508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0508-y