Abstract
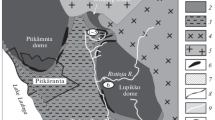
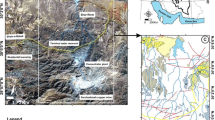
Acid mine waters (AMW) collected during high- and low-flow water conditions from the Lousal, Aljustrel, and São Domingos mining areas (Iberian Pyrite Belt) were physicochemically analyzed. Speciation calculation using PHREEQC code confirms the predominance of Men+ and Me–SO4 species in AMW samples. Higher concentration of sulfate species (Me–SO4) than free ion species (Men+, i.e., Al, Fe, and Pb) were found, whereas opposite behavior is verified for Mg, Cu, and Zn. A high mobility of Zn than Cu and Pb was identified. The sulfate species distribution shows that Fe3+–SO4 2−, SO4 2−, HSO4 −, Al–SO4, MgSO4 0, and CaSO4 0 are the dominant species, in agreement with the simple and mixed metal sulfates and oxy-hydroxysulphates precipitated from AMW. The saturation indices (SI) of melanterite and epsomite show a positive correlation with Cu and Zn concentrations in AMW, which are frequently retained in simple metal sulfates. Lead is well correlated with jarosite and alunite (at least in very acid conditions) than with simple metal sulfates. The Pb for K substitution in jarosite occurs as increasing Pb concentration in solution. Lead mobility is also controlled by anglesite precipitation (a fairly insoluble sulfate), where a positive correlation was ascertained when the SI approaches equilibrium. The zeta potential of AMW decreased as pH increased due to colloidal particles aggregation, where water species change from SO4 2− to OH− species during acid to alkaline conditions, respectively. The AMW samples were supersaturated in schwertmannite and goethite, confirmed by the Men+–SO4, Men+–Fe–O–OH, or Men+–S–O–Fe–O complexes identified by attenuated total reflectance infrared spectroscopy (ATR-IR). The ATR-IR spectrum of an AMW sample with pH 3.5 (sample L1) shows well-defined vibration plans attributed to SO4 tetrahedron bonded with Fe-(oxy)hydroxides and the Men+ sorbed by either SO4 or Fe-(oxy)hydroxides. For samples with lower pH values (pH ~ 2.5—samples SD1 and SD4), the vibration plans attributed to Men+ sorption are not evidenced, indicating its release in solution. The sorption of heavy metals on the first precipitated simple metal sulfates was ascertained by scanning electron microscopy coupled with X-ray spectrometry (SEM-EDX), where X-ray maps of Cu and Zn confirm a distribution of both metals in the melanterite structure.











Similar content being viewed by others
References
Alpers CN, Nordstrom DK (1999) Geochemical modelling of water-rock interactions in mining environments. In: Plumlee GS, Logsdon MJ (eds) The environmental geochemistry of mineral deposits, part a: processes, methods, and health issues, Rev Econ Geol Vol. 6A. Society of Economic Geology, Littleton, CO, pp. 289–323
Alpers CN, Nordstrom DK, Thompson JM (1994) Seasonal variations of Zn/Cu ratios in acid mine water from Iron Mountain, California. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation, ACS Symp Ser. 550. American Chemical Society, Washington DC, pp. 324–344
Andrade RFD, Schermerhorn LJG (1971) Aljustrel e Gavião. I Congresso Hispano-Luso-Americano de Geologia Económica, Livro-Guia n°4. Direcção-Geral de Minas e Serviços Geológicos, Lisboa, pp. 32–59
Ball JW, Nordstro, DK (1991) WATEQ4F-User’s manual with revised thermodynamic data base and test cases for calculating speciation of major, trace and redox elements in natural waters. U.S. Geological Survey, Open-File Report 90–129, 185 pp.
Barriga, FJAS (1983) Hydrothermal metamorphism and ore genesis at Aljustrel, Iberian Pyrite Belt. University of Ontario, Canada, Ph.D. Thesis, 368p
Barriga FJAS, Carvalho D, Ribeiro A (1997) Introduction to the Iberian Pyrite Belt. In: Barriga FJAS, Carvalho D (eds). Geology and VMS deposits of the Iberian Pyrite Belt. Neves Corvo Field Conference, Guidebook Series, Society of Economic Geologists 27:1–20
Barriga FJAS, Fyfe WS (1988) Giant pyritic base-metal deposits: the example of Feitais (Aljustrel, Portugal). Chem Geol 69(3–4):331–343. doi:10.1016/0009-2541(88)90044-7
Barriga FJAS, Fyfe WS (1998) Multi-phase water-rhyolite interaction and ore fluid generation at Aljustrel, Portugal. Mineral Deposita 33:88–207. doi:10.1007/s001260050140
Bigham JM, Nordstrom DK (2000) Iron and aluminium hydroxysulfates from acid sulfate waters. In: Alpers CN, Jambor JL, Nordstrom DK (eds) Sulfate minerals—crystallography, geochemistry, and environmental significance, Rev Mineral Geochem Vol. 40. The Mineralogical Society of America and Geochemical Society, Washington, DC, pp. 351–403
Bigham JM, Schwertmann U, Traina SJ, Winland RL, Wolf M (1996) Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim Cosmochim Ac 60:2111–2121. doi:10.1016/0016-7037(96)00091-9
Bishop JL, Murad E (1996) Schwertmannite on Mars? Spectroscopic analyses of schwertmannite, its relationship to other ferric minerals, and its possible presence in the surface material of Mars. In: Dyar MD, McCammon C, Schaefer MW (eds) Mineral spectroscopy: a tribute to Roger G. Burns, special publication, vol Vol. 5. Geochemical Society, New York, pp. 337–358
Bobos I, Durães N, Noronha F (2006) Mineralogy and geochemistry of mill tailings impoundments from Algares (Aljustrel), Portugal: implications for acid sulphate mine waters formation. J Geochem Explor 88:1–5. doi:10.1016/j.gexplo.2005.08.004
Carvalho D (1971) Mina de S. Domingos. I Congresso Hispano-Luso-Americano de Geologia Económica, Livro-Guia n°4. Direcção-Geral de Minas e Serviços Geológicos, Lisboa, pp. 59–64
Carvalho D, Conde L, Enrile JH, Oliveira V, Schermerhorn LJGS (1976) Livro–Guia das excursões geológicas na Faixa Piritosa Ibérica. Comum Serv Geol Port Tomo 60:271–315
Cravotta CA (2008) Dissolved metals and associated constituents in abandoned coalmine discharges, Pennsylvania, USA. Part 2: geochemical controls on constituent concentrations. Appl Geochem 23(2):203–226. doi:10.1016/j.apgeochem.2007.10.003
Drever JI (1997) The geochemistry of natural waters: surface and groundwater environments, 3rd edn. Prentice-Hall, Upper Saddle River
Durães N (2011) Geoquímica dos metais bivalentes tóxicos no sistema solos - sedimentos - águas - plantas em zonas contaminadas áridas e semi-áridas. Ph.D. Thesis, Faculdade de Ciências da Universidade do Porto, 289 pp.
Dzombak DA, Morel FMM (1990) Surface complexation modelling: hydrous ferric oxide. John Wiley & Sons, New York
Ferreira da Silva E, Cardoso Fonseca E, Matos JX, Patinha C, Reis P, Santos Oliveira JM (2005) The effect of unconfined mine tailings on the geochemistry of soils, sediments and surface waters of the Lousal area (Iberian Pyrite Belt, southern Portugal). Land Degrad Dev 16:213–228. doi:10.1002/ldr.659
Ferreira da Silva E, Patinha C, Reis P, Cardoso Fonseca E, Matos JX, Barrosinho J, Santos Oliveira JM (2006) Interaction of acid mine drainage with waters and sediments at the corona stream, Lousal mine (Iberian Pyrite Belt, southern Portugal). Environ Geol 50:1001–1013. doi:10.1007/s00254-006-0273-6
Ferreira da Silva E, Bobos I, Matos JX, Patinha C, Reis PA, Cardoso Fonseca E (2009) Mineralogy and geochemistry of trace metals and REE in volcanic massive sulfide host rocks, stream sediments, stream waters and acid mine drainage from the Lousal mine area (Iberian Pyrite Belt, Portugal). Appl Geochem 24:383–401. doi:10.1016/apgeochem.2008.12.001
Ficklin WH, Plumlee GS, Smith KS, McHugh JB (1992) Geochemical classification of mine drainages and natural drainages in mineralized areas. Proceedings of the 7th International Symposium on Water-Rock Interaction, Balkema, Rotterdam, pp 381–384
Gaspar O (1996) Microscopia e petrologia de minérios, aplicada à génese, exploração e mineralurgia dos sulfuretos maciços dos jazigos de Aljustrel e Neves Corvo. Est. Notas Trab Inst Geol Min Tomo 38:3–195
Hammarstrom JM, Seal RR II, Meier AL, Kornfeld JM (2005) Secondary sulfate minerals associated with acid mine drainage in the eastern US: recycling of metals and acidity in surficial environments. Chem Geol 215:407–431. doi:10.1016/j.chemgeo.2004.06.053
Hug SJ (1997) In situ Fourier transform infrared measurements of sulfate adsorption on hematite in aqueous solutions. J Colloid Interf Sci 188:415–422. doi:10.1006/jcis.1996.4755
Jambor JL, Blowes DW (1998) Theory and applications of mineralogy in environmental studies of sulphide-bearing mine wastes. In: Cabril LJ, Vaughan DJ (eds). Modern approaches to ore and environmental mineralogy, Short Courses Series Vol. 27, Mineralogical Association of Canada, pp 367–401
Jambor JL, Nordstrom DK, Alpers CN (2000) Metal-sulfate salts from sulphide mineral oxidation. In: Alpers CN, Jambor JL, Nordstrom DK (eds) Sulfate minerals—crystallography, geochemistry, and environmental significance, Rev Min Geochem Vol. 40. The Mineralogical Society of America and Geochemical Society, Washington, DC, pp. 303–350
Janzen MP, Nicholson RV, Scharer JM (2000) Pyrrhotite reaction kinetics: reaction rates for oxidation by oxygen, ferric iron, and for nonoxidative dissolution. Geochim Cosmochim Ac 64:1511–1522. doi:10.1016/S0016-7037(99)00421-4
Jennings SR, Dollhopf DJ, Inskeep WP (2000) Acid production from sulphide minerals using hydrogen peroxide weathering. Appl Geochem 15:235–243. doi:10.1016/S0883-2927(99)00041-4
Jerz JK, Rimstidt JD (2003) Efflorescent iron sulfate minerals: Paragenesis, relative stability, and environmental impact. Am Mineral 88:1919–1932
Kimball BA (1994) Seasonal variation in metal concentrations in a stream affected by acid mine drainage, St. Kevin Gulch, Colorado. In: Filipek LH, Plumlee GS (eds) The environmental geochemistry of mineral deposits, part B: case studies and research topics, Rev Econ Geol Vol. 6B. Society of Economic Geology, Littleton, CO, pp. 467–477
Lambert DC, McDonough KM, Dzombak DA (2004) Long-term changes in quality of discharge water from abandoned underground coal mines in Uniontown Syncline, Fayette County, PA, USA. Water Res 38(2):277–288. doi:10.1016/j.watres.2003.09.017
Lottermoser BG (2007) Mine wastes. Characterization, treatment, environmental impacts, 2nd edn. Springer-Verlag, Berlin
Matzke K (1971) Mina do Lousal. Livro-Guia n°4. I Congresso Hispano-Luso-Americano de Geologia Económica. Direcção-Geral de Minas e Serviços Geológicos, Lisboa, pp. 25–32
McCleskey RB (2013) New method for electrical conductivity temperature compensation. Environ Sci Technol 47:9874–9881. doi:10.1021/es402188r
McKibben MA, Tallant BA, del Angel JK (2008) Kinetics of inorganic arsenopyrite oxidation in acidic aqueous solutions. Appl Geochem 23:121–135. doi:10.1016/j.apgeochem.2007.10.009
Millero F (2001) Speciation of metals in natural waters. Geochem T 2:56–64. doi:10.1039/b104809k
Monterroso C, Alvarez E, Macías F (1994) Speciation and solubility control of Al and Fe in minesoil solutions. Sci Total Environ 158:31–43. doi:10.1016/0048-9697(94)90042-6
Nicholson RV, Scharer JM (1994) Laboratory studies of pyrrhotite oxidation kinetics. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation. American Chemical Society Symposium Series, Washington, DC, pp. 14–30
Nordstrom DK (1982) Aqueous pyrite oxidation and the consequent formation of secondary iron minerals. In: Kittrick JA, Fanning DS, Hossner LR (eds) Acid sulfate weathering. Soil Science Society of America, Special Publication 10, Madison, Wiscosin, pp. 37–56
Nordstrom DK (2009) Acid rock drainage and climate change. J Geochem Explor 100:97–104. doi:10.1016/j.gexplo.2008.08.002
Nordstrom DK (2011) Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface waters. Appl Geochem 26:1777–1791. doi:10.1016/j.apgeochem.2011.06.002
Nordstrom DK, Alpers CN (1999) Geochemistry of acid mine waters. In: Plumlee GS, Logsdon MJ (eds) The environmental geochemistry of mineral deposits, part a: processes, methods, and health issues, Rev Econ Geol Vol. 6A. Society of Economic Geology, Littleton, CO, pp. 133–160
Nordstrom DK, Ball JW (1989) Mineral saturation states in natural waters and their sensitivity to thermodynamic and analytical errors. Sci Géol Bull 42:269–280
Oliveira JT, Pereira Z, Carvalho P, Pacheco N, Korn D (2004) Stratigraphy of the tectonically imbricated lithological succession of the Neves Corvo mine area, Iberian Pyrite Belt, Portugal. Mineral Deposita 39:422–436. doi:10.1007/s00126-004-0415-2
Parkhurst DL, Appelo CAJ (2013) Description of input and examples for PHREEQC version 3--A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. Geological Survey Water-Resources Investigations. Chapter 43 of Section A, Groundwater Book 6, Modeling Techniques: Techniques and Methods 6–A43.
Peak D, Ford RG, Sparks DL (1999) An in situ ATR-FTIR investigation of sulfate bonding mechanisms on goethite. J Colloid Interf Sci 218:289–299. doi:10.1006/jcis.1999.6405
Peretyazhko T, Zachara JM, Boily J-F, Xia Y, Gassman PL, Arey BW, Burgos WD (2009) Mineralogical transformations controlling acid mine drainage chemistry. Chem Geol 262:169–178. doi:10.1016/j.chemgeo.2009.01.017
Rimstidt JD, Chermak JA, Gagen PM (1994) Rates of reaction of galena, sphalerite, chalcopyrite, and arsenopyrite with Fe(III) in acidic solutions. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation, ACS Symposium Series 550. American Chemical Society, Washington DC, pp. 2–13
Romero A, González I, Galán E (2006) The role of efflorescent sulfates in the storage of trace elements in stream waters polluted by acid mine-drainage: the case of Peña del Hierro, southwestern Spain. Can Mineral 44:1431–1446. doi:10.2113/gscanmin.44.6.1431
Sánchez España J, López Pamo E, Santofimia E, Aduvire O, Reyes J, Barettino D (2005) Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications. Appl Geochem 20:1320–1356. doi:10.1016/j.apgeochem.2005.01.011
Sánchez España J, López Pamo E, Santofimia Pastor E, Reyes Andrés J, Martín Rubí JA (2006) The removal of dissolved metals by hydroxysulphate precipitates during oxidation and neutralization of acid mine waters, Iberian Pyrite Belt. Aquat Geochem 12:269–298. doi:10.1007/s10498-005-6246-7
Schermerhorn LJG (1971) A Faixa Piritosa do Sul de Portugal. I Congresso Hispano-Luso-Americano de Geologia Económica, Livro-Guia n°4. Direcção-Geral de Minas e Serviços Geológicos, Lisboa, pp. 15–25
Simpson SL, Vardanega CR, Jarolimek C, Jolley DF, Angel BM, Mosley LM (2014) Metal speciation and potential bioavailability changes during discharge and neutralization of acidic drainage water. Chemosphere 103:172–180. doi:10.1016/j.chemosphere.2013.11.059
Singer PC, Stumm W (1970) Acid mine drainage: the rate-determining step. Science 167:1121–1123. doi:10.1126/science.167.3921.1121
Shum M, Lavkulich L (1999) Speciation and solubility relationships of Al, Cu and Fe in solutions associated with sulfuric acid leached mine waste rock. Environ Geol 38:59–68. doi:10.1007/s002540050401
Stumm W (1992) Chemistry of the solid-water interface: processes at the mineral-water and particle-water interface in natural systems. John Wiley & Sons, New York
Stumm W, Morgan J (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. John Wiley and Sons, New York
Viollier E, Inglett PW, Hunter K, Roychoudhury AN, Van Cappellen P (2000) The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl Geochem 15:785–790. doi:10.1016/S0883-2927(99)00097-9
Walker FP, Schreibe ME, Rimstidt JD (2006) Kinetics of arsenopyrite oxidative dissolution by oxygen. Geochim Cosmochim Ac 70:1668–1676. doi:10.1016/j.gca.2005.12.010
Webb JS (1958) Observations on the geology and origin of the San Domingos pyrite deposit, Portugal. Comun Serv Geol Port 42:129–143
Weisener CG, Smart RSC, Gerson AR (2004) A comparison of the kinetics and mechanism of acid leaching of sphalerite containing low and high concentrations of iron. Int J Miner Process 74:239–249. doi:10.1016/S0016-7037(02)01276-0
Yunmei Y, Yongxuan Z, Zheinmin G, Christopher HG, Denxian L (2007) Rates of arsenopyrite oxidation by oxygen and Fe(III) at pH 1.8 – 12.6 and 15 – 45 °C. Envir Sci Tech 41:6460–6464. doi:10.1021/es070788m
Zhang GY, Peak D (2007) Studies of Cd(II)-sulfate interactions at the goethite-water interface by ATR-FTIR spectroscopy. Geochim Cosmochim Ac 71:2158–2169. doi:10.1016/j.gca.2006.12.020
Acknowledgements
Nuno Durães is grateful to the Fundação para a Ciência e a Tecnologia (Portugal) for the financial support in the framework of the PhD scholarship (SFRH/BD/22413/2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Durães, N., Bobos, I. & da Silva, E.F. Speciation and precipitation of heavy metals in high-metal and high-acid mine waters from the Iberian Pyrite Belt (Portugal). Environ Sci Pollut Res 24, 4562–4576 (2017). https://doi.org/10.1007/s11356-016-8161-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8161-4