Abstract
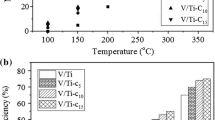
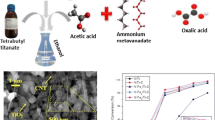
A series of V2O5/TiO2-carbon nanotube (CNT) catalysts were prepared and tested to decompose gaseous 1,2-dichlorobenzene (1,2-DCBz). Several physicochemical methods, including nitrogen adsorption, scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and H2 temperature-programmed reduction (TPR) were employed to characterise their physicochemical properties. To better understand the effect of CNT properties on the reactivity of V2O5/TiO2-CNT catalysts, the 1,2-DCBz residue remaining in the off-gas and on the catalyst surface were both collected and analysed. The results indicate that the outer diameter and the surface functional groups (hydroxide radical and carboxyl) of CNTs significantly influence upon the catalytic activity of CNT-containing V2O5/TiO2 catalysts: the CNT outer diameter mainly affects the aggregation of CNTs and the π-π interaction between the benzene ring and CNTs, while the introduction of –OH and –COOH groups by acid treatment can further enlarge specific surface area (SSA) and contribute to a higher average oxidation state of vanadium (V aos) and supplemental surface chemisorbed oxygen (Oads). In addition, the enhanced mobility of lattice oxygen (Olatt) also improves the oxidation ability of the catalysts.







Similar content being viewed by others
Abbreviations
- Specific surface area:
-
SSA
- Average oxidation state of vanadium:
-
V aos
- Surface chemisorbed oxygen:
-
Oads
- Lattice oxygen:
-
Olatt
- Removal efficiency:
-
RE
- Decomposition efficiency:
-
DE
- Adsorption efficiency:
-
AE
References
Apul OG, Shao T, Zhang SJ, Karanfil T (2012) Impact of carbon nanotube morphology on phenanthrene adsorption. Environ Toxicol Chem 31:73–78. doi:10.1002/etc.705
Bertinchamps F, Gregoire C, Gaigneaux EM (2006a) Systematic investigation of supported transition metal oxide based formulations for the catalytic oxidative elimination of (chloro)-aromatics—part I: identification of the optimal main active phases and supports. Appl Catal B-Environ 66:1–9. doi:10.1016/j.apcatb.2006.02.011
Bertinchamps F, Gregoire C, Gaigneaux EM (2006b) Systematic investigation of supported transition metal oxide based formulations for the catalytic oxidative elimination of (chloro)-aromatics—part II: influence of the nature and addition protocol of secondary phases to VO x /TiO2. Appl Catal B-Environ 66:10–22. doi:10.1016/j.apcatb.2006.02.012
Bertinchamps F, Poleunis C, Gregoire C, Eloy P, Bertrand P, Gaigneaux EM (2008) Elucidation of deactivation or resistance mechanisms of CrO x , VO x , and MnO x supported phases in the total oxidation of chlorobenzene via ToF-SIMS and XPS analyses. Surf Interface Anal 40:231–236. doi:10.1002/sia.2627
Bertinchamps F, Treinen M, Blangenois N, Mariage E, Gaigneaux EM (2005) Positive effect of NO x on the performances of VO x /TiO2-based catalysts in the total oxidation abatement of chlorobenzene. J Catal 230:493–498. doi:10.1016/j.jcat.2005.01.009
Buekens A, Huang H (1998) Comparative evaluation of techniques for controlling the formation and emission of chlorinated dioxins/furans in municipal waste incineration. J Hazard Mater 62:1–33. doi:10.1016/S0304-3894(98)00153-8
Chen JY, Chen W, Zhu D (2008) Adsorption of nonionic aromatic compounds to single-walled carbon nanotubes: effects of aqueous solution chemistry. Environ Sci Technol 42:7225–7230. doi:10.1021/es801412j
Chen L, Li JH, Ge MF (2009) Promotional effect of Ce-doped V2O5-WO3/TiO2 with low vanadium loadings for selective catalytic reduction of NO x by NH3. J Phys Chem C 113:21177–21184. doi:10.1021/jp907109c
Chen W, Duan L, Zhu DQ (2007) Adsorption of polar and nonpolar organic chemicals to carbon nanotubes. Environ Sci Technol 41:8295–8300. doi:10.1021/es071230h
Chin S, Jurng J, Lee JH, Moon SJ (2009) Catalytic conversion of 1,2-dichlorobenzene using V2O5/TiO2 catalysts by a thermal decomposition process. Chemosphere 75:1206–1209. doi:10.1016/j.chemosphere.2009.02.015
Cho CH, Ihm SK (2002) Development of new vanadium-based oxide catalysts for decomposition of chlorinated aromatic pollutants. Environ Sci Technol 36:1600–1606. doi:10.1021/es015687h
Cieplik MK, De Jong V, Bozovic J, Liljelind P, Marklund S, Louw R (2006) Formation of dioxins from combustion micropollutants over MSWI fly ash. Environ Sci Technol 40:1263–1269. doi:10.1021/es0522251
Daifullah AAM, Girgis BS (2003) Impact of surface characteristics of activated carbon on adsorption of BTEX. Colloid Surface A 214:181–193. doi:10.1016/S0927-7757(02)00392-8
Everaert K, Baeyens J (2002) The formation and emission of dioxins in large scale thermal processes. Chemosphere 46:439–448. doi:10.1016/S0045-6535(01)00143-6
He C, Yu YK, Shen Q, Chen JS, Qiao NL (2014) Catalytic behavior and synergistic effect of nanostructured mesoporous CuO-MnO x -CeO2 catalysts for chlorobenzene destruction. Appl Surf Sci 297:59–69. doi:10.1016/j.apsusc.2014.01.076
Komarneni M, Sand A, Goering J, Burghaus U, Lu M, Veca LM, Sun YP (2009) Possible effect of carbon nanotube diameter on gas-surface interactions—the case of benzene, water, and n-pentane adsorption on SWCNTs at ultra-high vacuum conditions. Chem Phys Lett 476:227–231. doi:10.1016/j.cplett.2009.05.072
Krishnamoorthy S, Rivas JA, Amiridis MD (2000) Catalytic oxidation of 1,2-dichlorobenzene over supported transition metal oxides. J Catal 193:264–272. doi:10.1006/jcat.2000.2895
Li HF, Lu GZ, Dai QG, Wang YQ, Guo Y, Guo YL (2011a) Efficient low-temperature catalytic combustion of trichloroethylene over flower-like mesoporous Mn-doped CeO2 microspheres. Appl Catal B-Environ 102:475–483. doi:10.1016/j.apcatb.2010.12.029
Li Q, Yang HS, Qiu FM, Zhang XB (2011b) Promotional effects of carbon nanotubes on V2O5/TiO2 for NO x removal. J Hazard Mater 192:915–921. doi:10.1016/j.jhazmat.2011.05.101
Li WZ, Gao F, Li Y, Walter ED, Liu J, Peden CHF, Wang Y (2015) Nanocrystalline anatase titania-supported vanadia catalysts: facet-dependent structure of vanadia. J Phys Chem C 119:15094–15102. doi:10.1021/acs.jpcc.5b01486
Lichtenberger J, Amiridis MD (2004a) Catalytic oxidation of chlorinated benzenes over V2O5/TiO2 catalysts. J Catal 223:296–29\. doi:10.1016/j.jcat.2004.01.032
Lichtenberger J, Amiridis MD (2004b) Deactivation of V2O5/TiO2 catalysts during the oxidation of meta-dichlorobenzene in the presence of methyl-naphthalene. Catal Today 98:447–453. doi:10.1016/j.cattod.2004.08.001
Liu Y, Wu WC, Guan YJ, Ying PL, Li C (2002) FT-IR spectroscopic study of the oxidation of chlorobenzene over Mn-based catalyst. Langmuir 18:6229–6232. doi:10.1021/la020125c
Long RQ, Yang RT (2001) Carbon nanotubes as superior sorbent for dioxin removal. J Am Chem Soc 123:2058–2059. doi:10.1021/ja003830l
Lu C, Su F, Hu S (2008) Surface modification of carbon nanotubes for enhancing BTEX adsorption from aqueous solutions. Appl Surf Sci 254:7035–7041. doi:10.1016/j.apsusc.2008.05.282
Mao D, He F, Zhao P, Liu ST (2015) Enhancement of resistance to chlorine poisoning of Sn-modified MnCeLa catalysts for chlorobenzene oxidation at low temperature. RSC Adv 5:10040–10047. doi:10.1039/c4ra15059g
Mastral AM, Garcia T, Callen MS, Navarro MV, Galban J (2001) Assessement of phenanthrene removal from hot gas by porous carbons. Energ Fuel 15:1–7. doi:10.1021/ef0001116g
McKay G (2002) Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: review. Chem Eng J 86:343–368. doi:10.1016/S1385-8947(01)00228-5
Murillo R, Garcia T, Aylon E, Callen MS, Navarro MV, Lopez JM, Mastral AM (2004) Adsorption of phenanthrene on activated carbons: breakthrough curve modeling. Carbon 42:2009–2017. doi:10.1016/j.carbon.2004.04.001
National Data (in Chinese) (2014) http://data.stats.gov.cn/easyquery.htm?cn=C01.
Nie AM, Yang HS, Li Q, Fan XY, Qiu FM, Zhang XB (2011) Catalytic oxidation of chlorobenzene over V2O5/TiO2–carbon nanotubes. Composites Industrial & Engineering Chemistry Research 50:9944–9948. doi:10.1021/ie200569a
Oh J-E, Gullett B, Ryan S, Touati A (2004) Chlorobenzenes, chlorophenols, PAHs and low chlorinated dioxin/furan as post-boiler toxicity indicators in municipal solid waste incinerators. Organohalogen Compd 66:777–782
Pan B, Lin DH, Mashayekhi H, Xing BS (2008) Adsorption and hysteresis of bisphenol A and 17 alpha-ethinyl estradiol on carbon nanomaterials. Environ Sci Technol 42:5480–5485. doi:10.1021/es8001184
Pan B, Xing BS (2008) Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ Sci Technol 42:9005–9013. doi:10.1021/es801777n
Standard for pollution control on the municipal solid waste incineration (in Chinese) (2014) http://kjs.mep.gov.cn/hjbhbz/bzwb/gthw/gtfwwrkzbz/201405/t20140530_276307.htm.
Tian W, Yang HS, Fan XY, Zhang XB (2010) Low-temperature catalytic oxidation of chlorobenzene over MnO x /TiO2-CNTs nano-composites prepared by wet synthesis methods. Catal Commun 11:1185–1188. doi:10.1016/j.catcom.2010.06.010
Wang Q-l, Huang Q-x, Wu H-f, Lu S-y, Wu H-l, Li X-d, Yan J-h (2016) Catalytic decomposition of gaseous 1,2-dichlorobenzene over CuO x /TiO2 and CuO x /TiO2-CNTs catalysts: mechanism and PCDD/Fs formation. Chemosphere 144:2343–2350. doi:10.1016/j.chemosphere.2015.10.097
Yang K, Zhu LZ, Xing BS (2006) Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ Sci Technol 40:1855–1861. doi:10.1021/es052208w
Yang RT, Long RQ, Padin J, Takahashi A, Takahashi T (1999) Adsorbents for dioxins: a new technique for sorbent screening for low-volatile organics. Ind Eng Chem Res 38:2726–2731. doi:10.1021/ie990170o
Yang Y et al (2015) Ball milling synthesized MnO x as highly active catalyst for gaseous POPs removal: significance of mechanochemically induced oxygen vacancies. Environ Sci Technol 49:4473–4480. doi:10.1021/es505232f
Zhang DS, Shi LY, Fang JH, Li XK, Dai K (2005) Preparation and modification of carbon nanotubes. Mater Lett 59:4044–4047. doi:10.1016/j.matlet.2005.07.081
Zhang SJ, Shao T, Bekaroglu SSK, Karanfil T (2009) The impacts of aggregation and surface chemistry of carbon nanotubes on the adsorption of synthetic organic compounds. Environ Sci Technol 43:5719–5725. doi:10.1021/es900453e
Acknowledgment
This project is supported by the National Natural Science Foundation of China (51276162), the Zhejiang Provincial Natural Science Foundation of China (R14E060001), the Doctoral Program of Higher Education (20130101110097) and the Program of Introducing Talents of Discipline to University (B08026).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Suresh Pillai
Rights and permissions
About this article
Cite this article
Du, C., Wang, Q., Peng, Y. et al. Catalytic oxidation of 1,2-DCBz over V2O5/TiO2-CNTs: effect of CNT diameter and surface functional groups. Environ Sci Pollut Res 24, 4894–4901 (2017). https://doi.org/10.1007/s11356-016-8075-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8075-1