Abstract
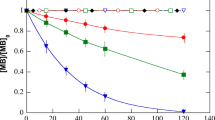
Acetaminophen (ACT) is one of the most frequently detected pharmaceuticals in aqueous environments, and treatment of ACT were generally carried out by photocatalytic degradations under high energy UV irradiation. In this study, potassium ferricyanide was utilized as a quadruple-elemental dopant in a TiO2 photocatalyst in order to enhance its visible-light activity. Two critical parameters (amounts of dopants and durations of calcination) of the synthesis of the photocatalyst by a sol–gel method were systematically evaluated. Crystal structure of the doping TiO2 was examined by X-ray diffraction while the effects of the two parameters on the photocatalytic activity were elucidated by various characterizations. Increasing the amount of dopant or the duration of calcination red-shifted the UV–vis DRS of the doped TiO2. The estimated band gap energy of the doped TiO2 decreased slightly as the amount of dopant increased, but it increased as the duration of calcination increased. The FT-IR yielded characteristic peaks that revealed the effects of the two parameters, whereas the SEM images revealed the morphological evolutions of each effect. The photocatalyst, synthesized at optimum conditions was able to remove 99.1 % acetaminophen with rate constant of 7.9 × 10−3 min−1, which was 4.88 times greater than virgin TiO2. In general, this study not only optimized synthetic conditions of the new visible-light active photocatalyst for ACT degradation but also presented characterizations conducted by SEM, XRD, UV–vis DRS, and FTIR to elucidate the relationship between modified structure and the photocatalytic activity.

Effects of doping amounts of K3[Fe(CN)6] and calcunation duration on visible light absorbance of TiO2 photocatalysts









Similar content being viewed by others
References
Abellán MN, Dillert R, Giménez J, Bahnemann D (2009) Evaluation of two types of TiO2-based catalysts by photodegradation of DMSO in aqueous suspension. J Photochem Photobiol A Chem 202:164–171. doi:10.1016/j.jphotochem.2008.11.020
Akpan UG, Hameed BH (2009) Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J Hazard Mater 170:520–529. doi:10.1016/j.jhazmat.2009.05.039
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269–271. doi:10.1126/science.1061051
Bessekhouad Y, Robert D, Weber JV, Chaoui N (2008) Effect of alkaline-doped TiO2 on photocatalytic efficiency. J Photochem Photobiol A 167:49–57. doi:10.1016/j.jphotochem.2003.12.001
Braslavsky SE, Braun AM, Cassano AE, Emeline AV, Litter MI, Palmisano L, Parmon VN, Serpone N (2011) Glossary of terms in photocatalysis and radiation catalysis (IUPAC recommendations 2011). Pure Appl Chem 83931–1014.doi:10.1351/PAC-REC-09-09-36
Chen LC, Huang CM, Tsai FR (2007a) Characterization and photocatalytic activity of K+-doped TiO2 photocatalysts. J Mol Catal A Chem 265:133–140. doi:10.1016/j.molcata.2006.10.011
Chen D, Jiang Z, Geng J, Wang Q, Yang D (2007b) Carbon and nitrogen codoped TiO2 with enhanced visible-light photocatalytic activity. Ind Eng Chem Res 46:2741–2746. doi:10.1021/ie061491k
Coates J (2000) In: Meyers R (ed) Encyclopedia of analytical chemistry. John Wiley & Sons Ltd., Chichester, pp 10815–10837
Daghrir R, Drogui P, Robert D (2013) Modified TiO2 for environmental photocatalytic applications: a review. Ind Eng Chem Res 52:3581–3599. doi:10.1021/ie303468t
Dalida MLP, Amer KMS, Su CC, Lu MC (2014) Photocatalytic degradation of acetaminophen in modified TiO2 under visible irradiation. Environ Sci Pollu Res 21:1208–1216. doi:10.1007/s11356-013-2003-4
de Luna MDG, Lin JCT, Gotostos MJN, Lu MC (2016) Photocatalytic oxidation of acetaminophen using carbon self-doped titanium dioxide, Sustain Environ Res 26 In Press, Corrected Proof, Available online 21 April 2016. 1–7. doi:10.1016/j.serj.2016.02.001
Fujishima A, Rao TN, Tryk DA (2000) Titanium dioxide photocatalysis. J Photochem Photobiol C Photochem Rev 11–21. doi:10.1016/S1389-5567(00)00002-2
Gamage McEvoy J, Cui W, Zhang Z (2013) Degradative and disinfective properties of carbon-doped anatase–rutile TiO2 mixtures under visible light irradiation. Catal Today 207:191–199. doi:10.1016/j.cattod.2012.04.015
Girish Kumar S, Gomathi Devi L (2011) Review of modified TiO2 photocatalysis under UV/visible light: selected results and related mechanisms on interfacial charge carrier transfer dynamics. J Phys Chem A 115:13211–13241. doi:10.1021/jp204364a
Gotostos MJN, Su CC, de Luna MDG, Lu MC (2014) Kinetic study of ACT degradation by visible light photocatalysis. J Environ Sci Health A 49:892–899. doi:10.1080/10934529.2014.894310
Hung WC, Fu SH, Tseng JJ, Chu H, Ko TH (2007) Study on photocatalytic degradation of gaseous dichloromethane using pure and iron ion-doped TiO2 prepared by the sol–gel method. Chemosphere 66:2142–2151. doi:10.1016/j.chemosphere.2006.09.037
Ihara T, Miyoshi M, Iriyama Y, Matsumoto O, Sugihara S (2003) Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Appl Catal B Environ 42:403–409. doi:10.1016/S0926-3373(02)00269-2
Irie H, Watanabe Y, Hashimoto K (2003) Carbon-doped anatase TiO2 powders as a visible-light sensitive photocatalyst. Chem Lett 32:772–773. doi:10.1246/cl.2003.772
Jo WK, Tayade RJ (2014) New generation energy-efficient light source for photocatalysis: LEDs for environmental applications. Ind Eng Chem Res 53:2073–2084. doi:10.1021/ie404176g
Kanakaraju D, Glass BD, Oelgemöller M (2014) Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ Chem Lett 1227–47. doi:10.1007/s10311-013-0428-0
Li D, Ohashi N, Hishita S, Kolodiazhnyi T, Haneda H (2005) Origin of visible-light-driven photocatalysis: a comparative study on N/F-doped and N–F-codoped TiO2 powders by means of experimental characterizations and theoretical calculations. J Solid State Chem 178:3293–3302. doi:10.1016/j.jssc.2005.08.008
Li K, Zheng H, Ivanov IN, Guthrie M, Xiao Y, Yang W, Tulk CA, Zhao Y, Mao HK (2013) K3Fe(CN)6: pressure-induced polymerization and enhanced conductivity. J Phys Chem C 117:24174–24180. doi:10.1021/jp407429z
Liu G, Zhang X, Xu Y, Niu X, Zheng L, Ding X (2005) The preparation of Zn2+-doped TiO2 nanoparticles by sol–gel and solid phase reaction methods respectively and their photocatalytic activities. Chemosphere 59:1367–1371. doi:10.1016/j.chemosphere.2004.11.072
Nadim AH, Al-Ghobashy MA, Nebsen M, Shehata MA (2015) Optimization of photocatalytic degradation of meloxicam using titanium dioxide nanoparticles: application to pharmaceutical wastewater analysis, treatment, and cleaning validation. Environ Sci Pollu Res 22:15516–15525. doi:10.1007/s11356-015-4713-2
Neville EM, Mattle MJ, Loughrey D, Rajesh B, Rahman M, Don MacElroy JM, Sullivan JA, Ravindranathan Thampi K (2012) Carbon-doped TiO2 and carbon, tungsten-codoped TiO2 through sol–gel processes in the presence of melamine borate: reflections through photocatalysis. J Phys Chem C 116:16511–16521. doi:10.1021/jp303645p
Ohno T, Miyamoto Z, Nishijima K, Kanemitsu H, Xueyuan F (2006) Sensitization of photocatalytic activity of S- or N-doped TiO2 particles by adsorbing Fe3+ cations. Appl Catal A General 302:62. doi:10.1016/j.apcata.2005.12.010
Orlikowski J, Tryba B, Ziebro J, Morawski AW, Przepiórski J (2012) A new method for preparation of rutile phase titania photoactive under visible light. Catal Commun 24:5–10. doi:10.1016/j.catcom.2012.02.027
Park Y, Kim W, Park H, Tachikawa T, Majima T, Choi W (2009) Carbon-doped TiO2 photocatalyst synthesized without using an external carbon precursor and visible light activity. Appl Catal B Environ 91:355–361. doi:10.1016/j.apcatb.2009.06.001
Roberts P, Thomas K (2006) The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci Total Environ 356:143–153. doi:10.1016/j.scitotenv.2005.04.031
Rokhina EV, Virkutyte J (2010) In: Virkutyte J, Varma RS, Jegatheesan V (eds) Treatment of micropollutants in water and wastewater. IWA Publishing, London, pp 361–424
Sakatani Y, Ando H, Okusako K, Koike H, Nunoshige J, Takata T, Kondo JN, Hara M, Domen KJ (2004) Metal ion and N co-doped TiO2 as a visible-light photocatalyst. J Mater Res 19:2100–2108. doi:10.1557/JMR.2004.0269
Sivakumar S, Krishna Pillai P, Mukundan P, Warrier KGK (2002) Sol–gel synthesis of nanosized anatase from titanyl sulfate. Mater Lett 57:330–335. doi:10.1016/S0167-577X(02)00786-3
Teoh WY, Scott JA, Amal R (2012) Progress in heterogeneous photocatalysis: From classical radical chemistry to engineering nanomaterials and solar reactors. J Phys Chem Lett 3:629–639. doi:10.1021/jz3000646
Tong T, Zhang J, Tian B, Chen F, He D (2008) Preparation of Fe3+-doped TiO2 catalysts by controlled hydrolysis of titanium alkoxide and study on their photocatalytic activity for methyl orange degradation. J Hazard Mater 155:572–579. doi:10.1016/j.jhazmat.2007.11.106
Treschev SY, Chou PW, Tseng YH, Wang JB, Perevedentseva EV, Cheng CL (2008) Photoactivities of the visible-light-activated mixed-phase carbon-containing titanium dioxide: the effect of carbon incorporation. Appl Catal B Environ 79:8–16. doi:10.1016/j.apcatb.2007.09.046
Trevisan V, Olivo A, Pinna F, Signoretto M, Vindigni F, Cerrato G, Bianchi CL (2014) C-N/TiO2 photocatalysts: effect of co-doping on the catalytic performance under visible light. Appl Catal B Environ 160–161:152–160. doi:10.1016/j.apcatb.2014.05.015
Tseng YH, Kuo CS, Huang CH, Li YY, Chou PW, Cheng CL, Wong MS (2006) Visible-light-responsive nano-TiO2 with mixed crystal lattice and its photocatalytic activity. Nanotechnol 17:2490–2497. doi:10.1088/0957-4484/17/10/009
Wang D, Xiao L, Luo Q, Li X, An J, Duan Y (2011) Highly efficient visible light TiO2 photocatalyst prepared by sol–gel method at temperatures lower than 300°C. J Hazard Mater 192:150–159. doi:10.1016/j.jhazmat.2011.04.110
Xiao X, Hu R, Liu C, Xing C, Qian C, Zuo X, Nan J, Wang L (2013) Facile large-scale synthesis of β-Bi2O3 nanospheres as a highly efficient photocatalyst for the degradation of acetaminophen under visible light irradiation. Appl Catal B Environ 140– 141:433–443. doi:10.1016/j.apcatb.2013.04.037
Xin B, Wang P, Ding D, Liu J, Ren Z, Fu H (2008) Effect of surface species on Cu-TiO2 photocatalytic activity. Appl Surf Sci 254:2569–2574. doi:10.1016/j.apsusc.2007.09.002
Yang J, Bai H, Jiang Q, Lian J (2008) Visible-light photocatalysis in nitrogen–carbon-doped TiO2 films obtained by heating TiO2 gel–film in an ionized N2 gas. Thin Solid Films 1736–1742. doi:10.1016/j.tsf.2007.05.034
Yang L, Yu LE, Ray MB (2008b) Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res 42:3480–3488. doi:10.1016/j.watres.2008.04.023
Yang X, Cao C, Erickson L, Hohn K, Maghirang R, Klabunde K (2009) Photo-catalytic degradation of Rhodamine B on C-, S-, N-, and Fe-doped TiO2 under visible-light irradation. Appl Catal B Environ 91:657–662. doi:10.1016/j.apcatb.2009.07.006
Yu C, Yu JC (2009) A simple way to prepare C-N-codoped TiO2 photocatalyst with visible-light activity. Catal Lett 129:462–470. doi:10.1007/s10562-008-9824-7
Acknowledgments
This research was supported by the Ministry of Science and Technology, Taiwan (NSC 101-2923-E-041-001-MY2) and the Engineering Research and Development for Technology—Department of Science and Technology, Philippines.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Suresh Pillai
Rights and permissions
About this article
Cite this article
Lin, J.CT., de Luna, M.D.G., Gotostos, M.J.N. et al. Effects of doping amounts of potassium ferricyanide with titanium dioxide and calcination durations on visible-light degradation of pharmaceuticals. Environ Sci Pollut Res 23, 22721–22733 (2016). https://doi.org/10.1007/s11356-016-7470-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7470-y