Abstract
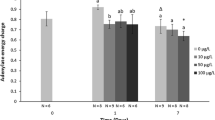
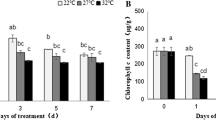
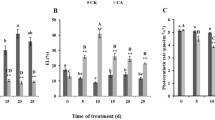
The aim of this study was to evaluate the coupled impact of an herbicide, ethofumesate, and temperature on the cellular energy metabolism of juvenile roach, especially on the glycolysis pathway. Juvenile roach were exposed to 0, 0.5, 5, and 50 μg/L of ethofumesate for 7 days in laboratory conditions at two temperatures (10 and 17 °C). The energy reserves (carbohydrate, lipid, and protein) were quantified, since the availability of substrates regulates the glycolysis. Then, the glycolysis was studied at the biochemical level by the measurement of the glycolytic flux and at the molecular level with the measurement of the relative expression of four genes encoding for glycolysis enzymes. This study revealed different effect of ethofumesate on the glycolysis pathway according to the temperature of exposure. Indeed, at 10 °C, it appeared that only the molecular regulation level was affected, whereas, at 17 °C, ethofumesate acted on the biochemical level. The differences observed between the two exposures imply the establishment of different strategies in order to maintain to cope with stress according to the temperature of exposure.






Similar content being viewed by others
References
Abulnaja KO, Tighe CR, Harwood JL (1992) Inhibition of fatty acid elongation provides a basis for the action of the herbicide, Ethofumesate, on surface wax formation. Phytochemistry 31:1155–1159
Angilletta MJ Jr, Niewiarowski PH, Navas CA (2002) The evolution of thermal physiology in ectotherms. J Therm Biol 27:249–268
Ba-Omar TA, Al-Jardani S, Victor R (2011) Effects of pesticide temephos on the gills of Aphanius dispar (Pisces: Cyprinodontidae). Tissue Cell 43:29–38
Becker AG, Moraes BS, Menezes CC, Loro VL, Santos DR, Reichert JM, Bladisserotto B (2009) Pesticide contamination of water alters the metabolism of juvenile silver catfish, Rhamdia quelen. Ecotoxicol Environ Saf 72:1734–1739
Begum G (2004) Carbofuran insecticide induced biochemical alterations in liver and muscle tissues of the fish Clarias batrachus (linn) and recovery response. Aquat Toxicol 66:83–92
Berenzen N, Kumke T, Schulz HK, Schulz R (2005) Macroinvertebrate community structure in agricultural streams: impact of runoff-related pesticide contamination. Ecotoxicol Environ Saf 60:37–46
Blanchoud H, Benoit M, Chevreuil M, Rat A, Ledoux E (2003) Modélisation du transfert de pesticides dans le bassin versant de la Marne. Presented at Colloque 2003 du PIREN-Seine, session 1: Agriculture et apports diffus
Bradford MM (1976) A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brinkmann M, Koglin S, Eisner B, Wiseman S, Hecker M, Eichbaum K, Thalmann B, Buchinger S, Reifferscheid G, Hollert H (2016) Characterisation of transcriptional responses to dioxins and dioxin-like contaminants in roach (Rutilus rutilus) using whole transcriptome analysis. Sci Total Environ 541:412–423
Bruslé J, Quignard JP (2013) Les gardons. In: Biologie des poissons d’eau douce européens, 2ème édn. TEC et DOC/Lavoisier, Paris, pp 235–245
Carvalho CDS, Fernandes MN (2008) Effect of copper on liver key enzymes of anaerobic glucose metabolism from freshwater tropical fish Prochilodus lineatus. Comp Biochem Physiol A 151:437–442
Charron L, Geffard O, Chaumot A, Coulaud R, Jaffal A, Gaillet V, Dedourge-Geffard O, Geffard A (2014) Influence of molting and starvation on digestive enzyme activities and energy storage in Gammarus fossarum. PLoS One 9:e96393
Ciacci C, Barmo C, Gallo G, Maisano M, Cappello T, D’Agata A, Leonzio C, Mauceri A, Fasulo S, Canesi L (2012) Effects of sublethal, environmentally relevant concentrations of hexavalent chromium in the gills of Mytilus galloprovincialis. Aquat Toxicol 120–121:109–118
De Boeck G, Vlaeminck A, Blust R (1997) Effects of sublethal copper exposure on copper accumulation, food consumption, growth, energy stores, and nucleic acid content in common carp. Arch Environ Contam Toxicol 33:415–422
De Boeck G, Van der Hen J, Hattink J, Blust R (2006) Swimming performance and energy metabolism of rainbow trout, common carp and gibel carp respond differently to sublethal copper exposure. Aquat Toxicol 80:92–100
Dedourge O, Geffard A, Amiard-Triquet C (2008) Origine des perturbations du métabolisme énergétique. In: Amiard JC, Amiard-Triquet C (eds) Les biomarqueurs dans l’évaluation de l’état écologique des milieux aquatiques. Tec et Doc/Lavoisier, Paris, pp 241–271
Dedourge-Geffard O, Charron L, Hofbauer C, Gaillet V, Palais F, Lacaze E, Geffard A, Geffard O (2013) Temporal patterns of digestive enzyme activities and feeding rate in gammarids (Gammarus fossarum) exposed to inland polluted waters. Ecotoxicol Environ Saf 97:139–146
Dragomirescu A, Raileanu L, Ababei L (1975) The effect of carbetox on glycolysis and the activity of some enzymes in carbohydrate metabolism in the fish and rat liver. Water Res 9:205–209
Durou C, Mouneyrac C, Pellerin J, Pery A (2008) Conséquences des perturbations du métabolisme énergétique. In: Amiard JC, Amiard-Triquet C (eds) Les biomarqueurs dans l’évaluation de l’état écologique des milieux aquatiques. Tec et Doc/Lavoisier, Paris, pp 273–294
Dutra BK, Fernandes FA, Oliveira GT (2008) Carbofuran-induced alterations in biochemical composition, lipoperoxidation, and Na+/K+ATPase activity of Hyalella pleoacuta and Hyalella curvispina in bioassays. Comp Biochem Physiol C 147:179–188
Dutra BK, Fernandes FA, Lauffer AL, Oliveira GT (2009) Carbofuran-induced alterations in the energy metabolism and reproductive behaviors of Hyalella castroi (Crustacea, Amphipoda). Comp Biochem Physiol C 149:640–646
Freed JM (1971) Properties of muscle phosphofructokinase of cold- and warm-acclimated Carassius auratus. Comp Biochem Physiol B 39:747–764
Geraudie P, Gerbron M, Hill E, Minier C (2010) Roach (Rutilus rutilus) reproductive cycle: a study of biochemical and histological parameters in a low contaminated site. Fish Physiol Biochem 36:767–777
Gurr MI, Harwoord JL (1991) Lipid biochemistry. An introduction, 4th edn. Chapman and Hall, London
Hepher B, Liao IC, Cheng SH, Hsieh CS (1983) Food utilization by red tilapia—effects of diet composition, feeding level and temperature on utilization efficiencies for maintenance and growth. Aquaculture 32:255–275
Herrero-Hernández E, Andrades MS, Álvarez-martín A, Pose-Juan E, Rodríguez-Cruz MS, Sánchez-Martín MJ (2013) Occurrence of pesticides and some of their degradation products in waters in a Spanish wine region. J Hydrol 486:234–245
Hohreiter DW, Reinert RE, Bush PB (1991) Effects of the insecticides carbofuran and fenvalerate on adenylate parameters in bluegill sunfish (Lepomis macrochirus). Arch Environ Contam Toxicol 21:325–331
Ibarz A, Blasco J, Beltrán M, Gallardo MA, Sánchez J, Sala R, Fernández-Borràs J (2005) Cold-induced alterations on proximate composition and fatty acid profiles of several tissues in gilthead sea bream (Sparus aurata). Aquaculture 249:477–486
Ibarz A, Blasco J, Sala-Rabanal M, Gallardo A, Redondo A, Fernández-Borràs J (2007a) Metabolic rate and tissue reserves in gilthead sea bream (Sparus aurata) under thermal fluctuations and fasting and their capacity for recovery. Can J Fish Aquat Sci 64:1034–1042
Ibarz A, Beltrán M, Fernández-Borràs J, Gallardo MA, Sánchez J, Blasco J (2007b) Alterations in lipid metabolism and use of energy depots of gilthead sea bream (Sparus aurata) at low temperatures. Aquaculture 262:470–480
Johnston IA, Dunn J (1987) Temperature acclimation and metabolism in ectotherms with particular reference to teleost fish. Symp Soc Exp Biol 41:67–93
Lauer MM, De Oliveira CB, Yano NLI, Bianchini A (2012) Copper effects on key metabolic enzymes and mitochondrial membrane potential in gills of the estuarine crab Neohelice granulate at different salinities. Comp Biochem Physiol C 156:140–147
Le Gal Y, Lagadic L, Le Bras S, Ramade F (1997) Charge énergétique en adénylates (CEA) et autres biomarqueurs associés au métabolisme énergétique. In: Lagadic L, Caquet T, Amiard JC, Ramade F (eds) Biomarqueurs en écotoxicologie : aspects fondamentaux. Masson, Paris, pp 241–285
Lemieux H (2007) Effets de la température sur le métabolisme mitochondrial cardiaque. Thesis, University of Québec
Leroy D, Haubruge E, De Pauw E, Thomé JP, Francis F (2010) Development of ecotoxicoproteomics on the freshwater amphipod Gammarus pulex: identification of PCB biomarkers in glycolysis and glutamate pathways. Ecotoxicol Environ Saf 73:343–352
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Long SM, Tull DL, Jeppe KJ, De Souza DP, Dayalan S, Pettigrove VJ, McConville MJ, Hoffmann AA (2015) A multi-platform metabolomics approach demonstrates changes in energy metabolism and the transsulfuration pathway in Chironomus tepperi following exposure to zinc. Aquat Toxicol 162:54–65
Lupatsch I, Kissil GW, Sklan D (2003) Defining energy and protein requirements of gilthead sea bream (Sparus aurata) to optimize feeds and feeding regimes. Isr J Aquacult 55:243–257
Moon TW (1988) Adaptation, constraint, and the function of the gluconeogenic pathway. Can J Zool 66:1059–1068
Neumann M, Liess M, Schulz R (2003) A qualitative sampling method for monitoring water quality in temporary channels or point sources and its application to pesticide contamination. Chemosphere 51:509–513
Oliveira C, Almeida J, Guilhermino L, Soares AMVM, Gravato C (2012) Acute effects of deltamethrin on swimming velocity and biomarkers of the common prawn Palaemon serratus. Aquat Toxicol 124–125:209–216
Pournourmohammadi S, Farzami B, Ostad SN, Azizi E, Abdollahi M (2005) Effects of malathion subchronic exposure on rat skeletal muscle glucose metabolism. Environ Toxicol Pharmacol 19:191–196
Rambabu JP, Rao MB (1994) Effect of an organochlorine and three organophosphate pesticides on glucose, glycogen, lipid, and protein contents in tissues of the freshwater snail Bellamya dissimilis (Müller). Bull Environ Contam Toxicol 53:142–148
Rao VJ (2006) Sublethal effects of an organophosphorous insecticide (RPR-II) on biochemical parameters of tilapia, Oreochromis mossambicus. Comp Biochem Physiol C 143:492–498
Reynders H, Bervoets L, Gelders M, De Coen WM, Blust R (2008) Accumulation and effects of metals in caged carp and resident roach along a metal pollution gradient. Sci Total Environ 391:82–96
Ribeiro S, Sousa JP, Nogueira AJA, Soares AMVM (2001) Effect of endosulfan and parathion on energy reserves and physiological parameters of the terrestrial isopod Porcellio dilatatus. Ecotoxicol Environ Saf 49:131–138
Roex EWM, Keijzers R, Van Gestel CAM (2003) Acetylcholinesterase and increased food consumption rate in the zebrafish, Danio rerio, after chronic exposure to parathion. Aquat Toxicol 64:451–460
Rome LC, Funke RP, Alexander RM, Lutz G, Aldridge H, Scott F, Freadman M (1988) Why animals have different muscle fibre types. Nature 335:824–827
Sancho E, Ferrando MD, Andreu E (1997) Inhibition of gill Na+, K+-ATPase activity in the eel, Anguilla anguilla, by fenitrothion. Ecotoxicol Environ Saf 38:132–136
Sancho E, Ferrando MD, Fernández C, Andreu E (1998) Liver energy metabolism of Anguilla anguilla after exposure to fenitrothion. Ecotoxicol Environ Saf 41:168–175
Sancho E, Villarroel MJ, Andreu E, Ferrando MD (2009) Disturbances in energy metabolism of Daphnia magna after exposure to tebuconazole. Chemosphere 74:1171–1178
Sébert P, Peragon J, Barroso JB, Simon B, Melendez-Hevia E (1998) High hydrostatic pressure (101 ATA) changes the metabolic design of yellow freshwater eel muscle. Comp Biochem Physiol B 121:195–200
Sharbidre AA, Metkari V, Patode P (2011) Effect of methyl parathion and chlorpyrifos on certain biomarkers in various tissues of guppy fish, Poecilia reticulata. Pestic Biochem Physiol 101:132–141
Somero GN (1969) Pyruvate kinase variants of the Alaska king crab: evidence for a temperature-dependent interconversion between two forms having distinct and adaptive kinetic properties. Biochem J 114:237–241
Somero GN (2004) Adaptation of enzymes to temperature: searching for basic “strategies”. Comp Biochem Physiol B 139:321–333
Somero GN, Hochachka PW (1968) The effect of temperature on catalytic and regulatory functions of pyruvate kinases of the rainbow trout and the Antarctic fish Trematomus bernacchii. Biochem J 110:395–400
Sullivan KM (1986) Physiology of feeding and starvation tolerance in overwintering freshwater fishes. In: Simenstad CA, Cailliet GM (eds) Contemporary studies on fish feeding: the proceedings of GUTSHOP’84, vol 7 of the series developments in environmental biology of fishes. W Junk Publishers, Pacific Grove, pp 259–268
The Pesticide Manual (2000) The pesticide manual, twelfth edition. CPC publications. British Crope Protection Council, The bath press, Bath, Farnham, Surrey, UK, pp 364–365
Tort L, Padrós F, Rotllant J, Crespo S (1998) Winter syndrome in the gilthead sea bream Sparus aurata. Immunological and histopathological features. Fish Shellfish Immunol 8:37–47
Tyler CR, Lange A, Paull GC, Katsu Y, Iguchi T (2007) The roach (Rutilus rutilus) as a sentinel for assessing endocrine disruption. Environ Sci 14:235–253
US EPA (2005) Reregistration eligibility decision for mancozeb. Office of Pesticides and Toxic Substances. Washington, DC
Van der Oost R, Van Gastel L, Worst D, Hanraads M, Satumalay K, Van Schooten FJ, Heida H, Vermeulen PE (1994) Biochemical markers in feral roach (Rutilus rutilus) in relation to the bioaccumulation of organic trace pollutants. Chemosphere 29:801–817
Verma AK, Pal AK, Manush SM, Das T, Dalvi RS, Chandrachoodan PP, Ravi PM, Apte SK (2007) Persistent sub-lethal chlorine exposure elicits the temperature induced stress responses in Cyprinus carpio early fingerlings. Pestic Biochem Physiol 87:229–237
Vidal T, Abrantes N, Gonçalves AMM, Gonçalves F (2011) Acute and chronic toxicity of Betanal®Expert and its active ingredients on nontarget aquatic organisms from different trophic levels. Environ Toxicol 27:537–548
Villarroel MJ, Sancho E, Andreu-Moliner E, Ferrando MD (2009) Biochemical stress response in tetradifon exposed Daphnia magna and its relationship to individual growth and reproduction. Sci Total Environ 407:5537–5542
Wallaert C, Babin PJ (1994) Effects of temperature variations on dietary lipid absorption and plasma lipoprotein concentrations in trout (Oncorhynchus mykiss). Comp Biochem Physiol B 109:473–487
Xu P, Wang Y, Zhang Y, Li J, Wang H (2014) Toxicity and bioaccumulation of ethofumesate enantiomers in earthworm Eisenia fetida. Chemosphere 112:163–169
Zhu W, Dang Z, Qiu J, Lv C, Jia G, Li L, Zhou Z (2007) Stereoselective toxicokinetics and tissue distribution of ethofumesate in rabbits. Chirality 19:632–637
Acknowledgments
This work was funded by the Champagne-Ardenne Region (RISKTOX Program) and by the French Ministry of Ecology and Sustainable Development (Programme 190 post grenelle DEVIL). Authors are grateful to the “Laboratoire Municipal et Régional de Reims” (France) for ethofumesate analyses in water samples. Authors are grateful to Marie Gacoin and Floriane Dantas, students in our laboratory, for the measurement of lipids and carbohydrates. Authors are grateful to laboratory technicians (Laurence Delahaut and Isabelle Bonnard) for their help for fish maintenance and dissections during ethofumesate exposures.
During this project, all experiments were conducted in accordance with the Commission recommendation 2007/526/EC on revised guidelines for the accommodation and care of animals used for experimental and other scientific purposes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Maes, V., Betoulle, S., Geffard, A. et al. Aerobic and anaerobic energy production in juvenile roach (Rutilus rutilus): regulation of glycolytic process by ethofumesate at two temperatures. Environ Sci Pollut Res 24, 6853–6865 (2017). https://doi.org/10.1007/s11356-016-6661-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6661-x