Abstract
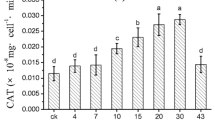
To identify a botanical algicide and elucidate the response of cyanobacteria to the extract from higher plants, the effects of garlic and garlic-derived diallyl trisulfide on Microcystis aeruginosa were studied. Effects were evaluated by changes in cell density, chlorophyll a, maximum effective quantum yield (Fv/Fm), effective quantum yield (YII), non-photochemical quenching (NPQ), and rapid light curves of M. aeruginosa. In addition, alkaline phosphatase activity (APA) was measured when M. aeruginosa was incubated with diallyl trisulfide. Results indicated that the inhibition by garlic and diallyl trisulfide was significant. The 120-h 50 % effective concentrations of garlic and diallyl trisulfide (EC50) were 0.75 g L−1 and 2.84 mg L−1, respectively. Moreover, the inhibitory rate increased with increasing concentration and the growth of M. aeruginosa was inhibited by 90.0 % at the highest concentrations. We also show that the response of M. aeruginosa to stress could involve both impairment of the photosynthetic center PSII and alteration of APA. For example, at high garlic concentration (2.0 g L−1), Fv/Fm significantly decreased from 0.501 to 0.084 (p < 0.05) after 120 h of exposure. Furthermore, the total APA was significantly decreased by exposure to a high diallyl trisulfide concentration after 24 h exposure. As new algal inhibitors, there are several advantages for their utilization, such as being common, cheap, non-toxic, and with high efficiency. It would be meaningful to further research on garlic as an environmentally friendly algicide.






Similar content being viewed by others
References
Beato VM, Sanchez AH, de Castro A, Montano A (2012) Effect of processing and storage time on the contents of organosulfur compounds in pickled blanched garlic. J Agric Food Chem 60(13):3485–3491
Berman T (1970) Alkaline phosphatases and phosphorus availability in Lake Kinneret. Limnol Oceanogr 15(5):663–674
Campbell D, Hurry V, Clarke AK, Gustafsson P, Oquist G (1998) Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev 62(3):667–683
Carmichael WW (2001) Health effects of toxin-producing cyanobacteria: ‘The CyanoHABs’. Human And Ecological Risk Assessment 7(5):1393–1407
Codd GA (2000) Cyanobacterial toxins, the perception of water quality, and the prioritisation of eutrophication control. Ecol Eng 16(1):51–60
Cornelissen G, VanNoort P, Parsons JR, Govers H (1997) Temperature dependence of slow adsorption and desorption kinetics of organic compounds in sediments. Environ Sci Technol 31(2):454–460
Domingos P, Rubim TK, Molica R, Azevedo S, Carmichael WW (1999) First report of microcystin production by picoplanktonic cyanobacteria isolated from a northeast Brazilian drinking water supply. Environ Toxicol 14(1):31–35
Drabkova M, Marsalek B, Admiraal W (2007) Photodynamic therapy against cyanobacteria. Environ Toxicol 22:112–115
Dziga D, Suda M, Bialczyk J, Czaja-Prokop U, Lechowski Z (2007) The alteration of Microcystis aeruginosa biomass and dissolved microcystin-LR concentration following exposure to plant-producing phenols. Environ Toxicol 22(4):341–346
Gross EM, Erhard D, Ivanyi E (2003) Allelopathic activity of Ceratophyllum demersum L. and Najas marina ssp intermedia (Wolfgang) Casper. Hydrobiologia 506(1–3):583–589
Gross EM, Meyer H, Schilling G (1996) Release and ecological impact of algicidal hydrolysable polyphenols in Myriophyllum spicatum. Phytochemistry 41(1):133–138
Gull I, Saeed M, Shaukat H, Aslam S M, Samra Z Q, Athar A M (2012) Inhibitory effect of Allium sativum and Zingiber officinale extracts on clinically important drug resistant pathogenic bacteria. Annals of Clinical Microbiology and Antimicrobials 11(8). doi:10.1186/1476-0711-11-8
Hehmann A, Kaya K, Watanabe MM (2002) Selective control of Microcystis using an amino acid-a laboratory assay. J Appl Phycol 14:85–89
Hong Y, Hu HY, Sakoda A, Sagehashi M (2009a) Isolation and characterization of antialgal allelochemicals from Arundo donax L. Aquat Toxicol 25(3):357–367
Hong Y, Hu HY, Xie X, Sakoda A, Sagehashi M, Li FM (2009b) Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquat Toxicol 91(3):262–269
Huang BQ, Ou LJ, Hong HS, Luo HW, Wang DZ (2005) Bioavailability of dissolved organic phosphorus compounds to typical harmful dinoflagellate Prorocentrum donghaiense Lu. Mar Pollut Bull 51(8–12):838–844
Jassby A, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21(4):540–547
Korner S, Nicklisch A (2002) Allelopathic growth inhibition of selected phytoplankton species by submerged macrophytes. J Phycol 38(5):862–871
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Kumar KS, Dahms HU, Lee JS, Kim HC, Lee WC, Shin KH (2014) Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicol Environ Saf 104:51–71
Lee JY, Gao Y (2012) Review of the application of garlic. Allium sativum, in aquaculture, Journal of the World Aquaculture Society 43:447–458
Lelkova E, Rulik M, Hekera P, Dobias P, Borovickova M, Poulickova A (2008) The influence of the coagulant PAX-18 on Planktothrix agardhii bloom in a shallow eutrophic fishpond. Fottea 8:147–154
Liu JS, Zhang H, Yang WD, Gao J, Ke Q (2004) Studies on biquaternary ammonium salt algicide for removing red tide algae. Mar Sci Bull 6:60–65
Magnusson M, Heimann K, Quayle P, Negri AP (2010) Additive toxicity of herbicide mixtures and comparative sensitivity of tropical benthic microalgae. Mar Pollut Bull 60(11):1978–1987
Meepagala KA, Schrader KK, Wedge DE, Duke SO (2005) Algicidal and antifungal compounds from the roots of Ruta graveolens and synthesis of their analogs. Phytochemistry 66(22):2689–2695
Militz TA, Southgate PC, Carton AG, Hutson KS (2014) Efficacy of garlic (Allium sativum) extract applied as a therapeutic immersion treatment for Neobenedenia sp. Management in aquaculture, Journal of Fish Diseases 37:451–461
Mulderij G, Mooij WM, Smolders A, Van Donk E (2005) Allelopathic inhibition of phytoplankton by exudates from Stratiotes aloides. Aquatic Botany 82(4):284–296
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64(13):3983–3998
Nakai S, Zou G, Okuda T, Tsai TY, Song X, Nishijima W, Okada M (2010) Anti-cyanobacterial allelopathic effects of plants used for artificial floating islands. Allelopathy Journal 26(1):113–121
Qiu HM, Geng JJ, Ren HQ, Xia XM, Wang XR, Yu Y (2013) Physiological and biochemical responses of Microcystis aeruginosa to glyphosate and its Roundup@ formulation. J Hazard Mater 248:172–176
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquatic Botany 82(3):222–237
Smith JL, Boyer GL, Zimba PV (2008) A review of cyanobacterial odorous and bioactive metabolites: impacts and management alternatives in aquaculture. Aquaculture 280(1–4):5–20
State Environmental Protection Administration (SEPA) (2002) Water and wastewater monitoring analysis method, 4th edn. China Environmental Science Press (in Chinese), Beijing
Wang ZC, Li DH, Qin HJ, Li YX (2012) An integrated method for removal of harmful cyanobacterial blooms in eutrophic lakes. Environ Pollut 160:34–41
Wu ZX, Gan NQ, Huang Q, Song LR (2007) Response of Microcystis to copper stress—do phenotypes of Microcystis make a difference in stress tolerance? Environ Pollut 147(2):324–330
Yi YL, Lei Y, Yin YB, Zhang HY, Wang GX (2012) The antialgal activity of 40 medicinal plants against Microcystis aeruginosa. Journal of Applied Phycology 24(4):847–856
Zhang H, Yang WD, Gao J, Liu JS (2003) Inhibition and elimination of chlorine dioxide on Phaeocystis globosa. Chin J Appl Ecol 14:1173–1176
Zhou LH, Zheng TL, Chen XH, Wang X, Chen SB, Tian Y, Hong HS (2008) The inhibitory effects of garlic (Allium sativum) and diallyl trisulfide on Alexandrium tamarense and other harmful algal species. Journal of Applied Phycology 20(4):349–358
Zhou LH, Zheng TL, Wang X, Ye JL, Tian Y, Hong HS (2007) Effect of five Chinese traditional medicines on the biological activity of a red-tide causing alga—Alexandrium tamarense. Harmful Algae 6(3):354–360
Zhou SQ, Shao YS, Gao NY, Deng Y, Qiao JL, Ou HS, Deng J (2013) Effects of different algaecides on the photosynthetic capacity, cell integrity and microcystin-LR release of Microcystis aeruginosa. Science of The Total Environment 463:111–119
Zhu JY, Liu BY, Wang J, Gao YN, Wu ZB (2010) Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion. Aquat Toxicol 98(2):196–203
Zou H, Pan G, Chen H, Yuan XZ (2006) Removal of cyanobacterial blooms in Taihu Lake using local soils. II Effective removal of Microcystis aeruginosa using local soils and sediments modified by chitosan Environmental Pollution 141(2):201–205
Acknowledgments
This research was supported by funding from the National Science and Technology Major Project for Water Pollution Control and Treatment (2012ZX07102-004). We also thank anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Wang, S., Wang, Y., Ma, X. et al. Effects of garlic and diallyl trisulfide on the growth, photosynthesis, and alkaline phosphatase activity of the toxic cyanobacterium Microcystis aeruginosa . Environ Sci Pollut Res 23, 5712–5720 (2016). https://doi.org/10.1007/s11356-015-5809-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5809-4