Abstract
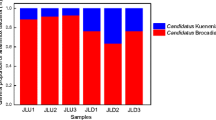
The anaerobic ammonium oxidation (anammox) process, which can simultaneously remove ammonium and nitrite, both toxic to aquatic animals, can be very important to the aquaculture industry. Here, the presence and activity of anammox bacteria in the sediments of four different freshwater aquaculture ponds were investigated by using Illumina-based 16S rRNA gene sequencing, quantitative PCR assays and 15N stable isotope measurements. Different genera of anammox bacteria were detected in the examined pond sediments, including Candidatus Brocadia, Candidatus Kuenenia and Candidatus Anammoxoglobus, with Candidatus Brocadia being the dominant anammox genus. Quantitative PCR of hydrazine synthase genes showed that the abundance of anammox bacteria ranged from 5.6 × 104 to 2.1 × 105 copies g−1 sediment in the examined ponds. The potential anammox rates ranged between 3.7 and 19.4 nmol N2 g−1 sediment day−1, and the potential denitrification rates varied from 107.1 to 300.3 nmol N2 g−1 sediment day−1. The anammox process contributed 1.2–15.3 % to sediment dinitrogen gas production, while the remainder would be due to denitrification. It is estimated that a total loss of 2.1–10.9 g N m−2 per year could be attributed to the anammox process in the examined ponds, suggesting that this process could contribute to nitrogen removal in freshwater aquaculture ponds.




Similar content being viewed by others
References
Arrigo KR (2005) Marine microorganisms and global nutrient cycles. Nature 437:349–355
Bao SD (ed) (2000) Chemical analysis for agricultural soil. China Agriculture Press, Beijing
Brandes JA, Devol AH, Deutsch C (2007) New developments in the marine nitrogen cycle. Chem Rev 107:577–589
Castine A, Erler DV, Trott LA, Paul NA, de Nys R, Eyre BD (2012) Denitrification and anammox in tropical aquaculture settlement ponds: an isotope tracer approach for evaluating N2 production. PLoS ONE 7, e42810. doi:10.1371/journal.pone.0042810
Dale OR, Tobias CR, Song B (2009) Biogeographical distribution of diverse anaerobic ammonium oxidizing (anammox) bacteria in Cape Fear River Estuary. Environ Microbiol 11:1194–1207
Dalsgaard T, Thamdrup B, Canfield DE (2005) Anaerobic ammonium oxidation (anammox) in the marine environment. Res Microbiol 156:457–464
Dang H, Chen R, Wang L, Guo L, Chen P, Tang Z, Tian F, Li S, Klotz MG (2010) Environmental factors shape sediment anammox bacterial communities in hypernutrified Jiaozhou Bay, China. Appl Environ Microbiol 76:7036–7047
de Paula Silva PH, McBride S, de Nys R, Paul NA (2008) Integrating filamentous ‘green tide’ algae into tropical pond-based aquaculture. Aquaculture 284:74–80
Eddy FB (2005) Ammonia in estuaries and effects on fish. J Fish Biol 67:1495–1513
Engström P, Dalsgaard T, Hulth S, Aller RC (2005) Anaerobic ammonium oxidation by nitrite (anammox): implications for N2 production in coastal marine sediments. Geochim Cosmochim Acta 69:2057–2065
Ettwig KF, van Alen T, van de Pas-Schoonen KT, Jetten MSM, Strous M (2009) Enrichment and molecular detection of denitrifying methanotrophic bacteria of the NC10 phylum. Appl Environ Microbiol 75:3656–3662
Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, Ravel J (2014) An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2:6. doi:10.1186/2049-2618-2-6
FAO (2014) The state of world fisheries and aquaculture 2014. Food and Agricultural Organization of the United Nations
Gori F, Tringe SG, Kartal B, Marchiori E, Jetten MSM (2011) The metagenomic basis of anammox metabolism in Candidatus ‘Brocadia fulgida’. Biochem Soc Trans 39:1799–1804
Harhangi HR, Le Roy M, van Alen T, Hu BL, Groen J, Kartal B, Tringe SG, Quan ZX, Jetten MSM, Op den Camp HJM (2012) Hydrazine synthase, a unique phylomarker to study the presence and biodiversity of anammox bacteria. Appl Environ Microbiol 78:752–758
Hira D, Toh H, Migita CT, Okubo H, Nishiyama T, Hattori M, Furukawa K, Fujii T (2012) Anammox organism KSU-1 expresses a NirK-type copper-containing nitrite reductase instead of a NirS-type with cytochrome cd1. FEBS Lett 586:1658–1663
Hou LJ, Zheng YL, Liu M, Gong J, Zhang XL, Yin GY, You L (2013) Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze Estuary. J Geophys Res Biogeosci 118:1237–1246
Hu BL, Shen LD, Xu XY, Zheng P (2011) Anaerobic ammonium oxidation (anammox) in different natural ecosystems. Biochem Soc Trans 39:1811–1816
Hu BL, Shen LD, Zheng P, Hu AH, Chen TT, Cai C, Liu S, Lou LP (2012a) Distribution and diversity of anaerobic ammonium-oxidizing bacteria in the sediments of the Qiantang River. Environ Microbiol Rep 4:540–547
Hu ZY, Speth DR, Francoijs KJ, Quan ZX, Jetten MSM (2012b) Metagenome analysis of a complex community reveals the metabolic blueprint of anammox bacterium “Candidatus Jettenia asiatica”. Front Microbiol 3:1–9
Hu BL, Shen LD, Liu S, Cai C, Chen TT, Kartal B, Harhangi HR, Op den Camp HJM, Lou LP, Xu XY, Zheng P, Jetten MSM (2013) Enrichment of an anammox bacterial community from a flooded paddy soil. Environ Microbiol Rep 5:483–489
Jetten MS, Lv N, Strous M, Kartal B, Keltjens JT, Op den Camp HJ (2009) Biochemistry and molecular biology of anammox bacteria. Crit Rev Biochem Mol Biol 44:65–84
Kartal B, Maalcke WJ, de Almeida NM, Cirpus I, Gloerich J, Geerts W, Op den Camp HJM, Harhangi HR, Janssen-Megens EM, Francoijs K, Stunnenberg HG, Keltjens JT, Jetten MSM, Strous M (2011) Molecular mechanism of anaerobic ammonium oxidation. Nature 479:127–130
Lam P, Lavik G, Jensen MM, van de Vossenberg J, Schmid M, Woebken D, Gutiérrez D, Amann R, Jetten MS, Kuypers MM (2009) Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc Natl Acad Sci U S A 106:4752–4757
Liang J, Sun S, Ji J, Wu H, Meng F, Zhang MR, Zheng XB, Wu CX, Zhang ZG (2014) Comparison of the rhizosphere bacterial communities of Zigongdongdou soybean and a high-methionine transgenic line of this cultivar. PLoS ONE 9, e103343. doi:10.1371/journal.pone.0103343
Liu JJ, Sui YY, Yu ZH, Shi Y, Chu HY, Jin J, Liu XB, Wang GH (2014) High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol Biochem 70:113–122
Lu SM, Liao MJ, Xie CX, He XG, Li DP, He LL, Chen J (2015) Seasonal dynamics of ammonia-oxidizing microorganisms in freshwater aquaculture ponds. Ann Microbiol 66:651–657
Mommsen TP, Walsh PJ (1992) Biochemical and environmental perspectives on nitrogen-metabolism in fishes. Experientia 48:583–593
Mulder A, Van de Graaf AA, Robertson LA, Kuenen JG (1995) Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol Ecol 16:177–183
Risgaard-Petersen N, Meyer RL, Schmidt M, Jetten MSM, Prast AE, Rysgaard S (2004) Anaerobic ammonia oxidation in an estuarine sediment. Aquat Microb Ecol 36:293–304
Schmid MC, Risgaard-Petersen N, van de Vossenberg J, Kuypers MMM, Lavik G, Petersen J, Hulth S, Thamdrup B, Canfield D, Dalsgaard T, Rysgaard S, Sejr MK, Strous M, Op den Camp HJM, Jetten MSM (2007) Anaerobic ammonium-oxidizing bacteria in marine environments: widespread occurrence but low diversity. Environ Microbiol 9:1476–1486
Schubert CJ, Durisch-Kaiser E, Wehrli B, Thamdrup B, Lam P, Kuypers MMM (2006) Anaerobic ammonium oxidation in a tropical freshwater system (Lake Tanganyika). Environ Microbiol 8:1857–1863
Shen LD, Liu S, Lou LP, Liu WP, Xu XY, Zheng P, Hu BL (2013) Broad distribution of diverse anaerobic ammonium-oxidising bacteria in Chinese agricultural soils. Appl Environ Microbiol 79:6167–6172
Shen LD, Liu S, Huang Q, Lian X, He ZF, Geng S, Jin RC, He YF, Lou LP, Xu XY, Zheng P, Hu BL (2014) Evidence for the co-occurrence of nitrite-dependent anaerobic ammonium and methane oxidation processes in a flooded paddy field. Appl Environ Microbiol 80:7611–7619
Shen LD, Wu HS, Gao ZQ, Xu XH, Chen TX, Liu S, Cheng HX (2015) Occurrence and importance of anaerobic ammonium-oxidising bacteria in vegetable soils. Appl Microbiol Biotechnol 99:5709–5718
Strous M, Fuerst JA, Kramer EHM, Logemann S, Muyzer G, van de Pas-Schoonen KT, Webb R, Kuenen JG, Jetten MSM (1999) Missing lithotroph identified as new planctomycete. Nature 400:446–449
Strous M, Pelletier E, Mangenot S, Rattei T, Lehner A, Taylor MW, Horn M, Daims H, Bartol-Mavel D, Wincker P, Barbe V, Fonknechten N, Vallenet D, Segurens B, Schenowitz-Truong C, Médigue C, Collingro A, Snel B, Dutilh BE, Op den Camp HJ, van der Drift C, Cirpus I, van de Pas-Schoonen KT, Harhangi HR, van Niftrik L, Schmid M, Keltjens J, van de Vossenberg J, Kartal B, Meier H, Frishman D, Huynen MA, Mewes HW, Weissenbach J, Jetten MS, Wagner M, Le Paslier D (2006) Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440:790–794
Sun W, Xu MY, Wu WM, Guo J, Xia CY (2014) Molecular diversity and distribution of anammox community in sediments of the Dongjiang River, a drinking water source of Hong Kong. J Appl Microbiol 116:464–476
Tal Y, Watts JE, Schreier HJ (2006) Anaerobic ammonium-oxidizing (anammox) bacteria and associated activity in fixed-film biofilters of a marine recirculating aquaculture system. Appl Environ Microbiol 72:2896–2904
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
van de Vossenberg J, Woebken D, Maalcke WJ, Wessels HJ, Dutilh BE, Kartal B, Janssen-Megens EM, Roeselers G, Yan J, Speth D, Gloerich J, Geerts W, van der Biezen E, Pluk W, Francoijs KJ, Russ L, Lam P, Malfatti SA, Tringe SG, Haaijer SC, Op den Camp HJ, Stunnenberg HG, Amann R, Kuypers MM, Jetten MS (2013) The metagenome of the marine anammox bacterium “Candidatus Scalindua profundal” illustrates the versatility of this globally important nitrogen cycle bacterium. Environ Microbiol 15:1275–1289
van Kessel MAHJ, Harhangi HR, van de Pas-Schoonen K, van de Vossenberg J, Flik G, Jetten MSM, Klaren PHM, Op den Camp HJM (2010) Biodiversity of N-cycle bacteria in nitrogen removing moving bed biofilters for freshwater recirculating aquaculture systems. Aquaculture 306:177–184
van Kessel MAHJ, Harhangi HR, Flik G, Jetten MSM, Klaren PHM, Op den Camp HJM (2011) Anammox bacteria in different compartments of recirculating aquaculture systems. Biochem Soc Trans 39:1817–1821
van Rijn J, Tal Y, Schreier HJ (2006) Denitrification in recirculating systems: theory and applications. Aquac Eng 34:364–376
Wang S, Zhu G, Peng Y, Jetten MS, Yin C (2012) Anammox bacterial abundance, activity, and contribution in riparian sediments of the Pearl River estuary. Environ Sci Technol 46:8834–8842
Wenk CB, Blees J, Zopfi J, Veronesi M, Bourbonnais A, Schubert CJ, Niemann H, Lehmann MF (2013) Anaerobic ammonium oxidation (anammox) bacteria and sulfide-dependent denitrifiers coexist in the water column of a meromictic south-alpine lake. Limnol Oceanogr 58:1–12
Woebken D, Lam P, Kuypers MMM, Naqvi SW, Kartal B, Strous M, Jetten MSM, Fuchs BM, Amann R (2008) A microdiversity study of anammox bacteria reveals a novel Candidatus Scalindua phylotype in marine oxygen minimum zones. Environ Microbiol 10:3106–3119
Wu S, Wang G, Angert ER, Wang W, Li W, Zou H (2012) Composition, diversity, and origin of the bacterial community in grass carp intestine. PLoS ONE 7, e30440. doi:10.1371/journal.pone.0030440
Yang XR, Li H, Nie SA, Su JQ, Weng BS, Zhu GB, Yao HY, Gilbert JA, Zhu YG (2015) Potential contribution of anammox to nitrogen loss from paddy soils in Southern China. Appl Environ Microbiol 81:938–947
Yoshinaga I, Amano T, Yamagishi T, Okada K, Ueda S, Sako Y, Suwa Y (2011) Distribution and diversity of anaerobic ammonium oxidation (anammox) bacteria in the sediment of a eutrophic freshwater lake, Lake Kitaura, Japan. Microbes Environ 26:189–197
Zhang Y, Ruan XH, Op den Camp HJM, Smits TJM, Jetten MSM, Schmid MC (2007) Diversity and abundance of aerobic and anaerobic ammonium-oxidizing bacteria in freshwater sediments of the Xinyi River (China). Environ Microbiol 9:2375–2382
Zhao Y, Xia Y, Kana TM, Wu Y, Li X, Yan X (2013) Seasonal variation and controlling factors of anaerobic ammonium oxidation in freshwater river sediments in the Taihu Lake region of China. Chemosphere 93:2124–2431
Zhou S, Borjigin S, Riya S, Terada A, Hosomi M (2014) The relationship between anammox and denitrification in the sediment of an inland river. Sci Total Environ 290:1029–1036
Zhu GB, Wang S, Wang Y, Wang C, Risgaard-Petersen N, Jetten MSM, Yin C (2011) Anaerobic ammonia oxidation in a fertilized paddy soil. ISME J 5:1905–1912
Zhu GB, Wang S, Wang W, Wang Y, Zhou J, Jiang B, Op den Camp HJM, Risgaard N, Schwark L, Peng Y, Hefting M, Jetten MSM, Yin C (2013) Hotspots of anaerobic ammonium oxidation at land–freshwater interfaces. Nat Geosci 6:103–107
Acknowledgments
This study was supported by the Natural Science Foundation of China (No. 41501261), the Natural Science Foundation of Jiangsu Province (No. BK20150893), State Key Laboratory of Atmospheric Boundary Layer Physics and Atmospheric Chemistry, Institute of Atmospheric Physics, Chinese Academy of Sciences, the Startup Foundation for Introducing Talent of NUIST (no. S8113112001), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Natural Science Foundation of China (31402348), and the Aquaculture Facilities Innovation Team Project (2011R50029).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Basic physiochemical parameters of the examined aquaculture ponds (DOC 35 kb)
Table S2
The numbers of initial sequences, sequences after chimera filter, planctomycete sequences, and anammox-related sequences in each sample obtained by Illumina-based 16S rRNA gene sequencing (DOC 31 kb)
Fig. S1
The four different aquaculture ponds examined in this study (DOC 34 kb)
Fig. S2
Neighbour-joining phylogenetic tree showing the phylogenetic affiliations of the anammox bacterial hzsA gene sequences recovered from qPCR. The bootstrap values were 1000 replicates, and the scale bar represents 5 % of the sequence divergence (DOC 28 kb)
Rights and permissions
About this article
Cite this article
Shen, Ld., Wu, Hs., Gao, Zq. et al. Evidence for anaerobic ammonium oxidation process in freshwater sediments of aquaculture ponds. Environ Sci Pollut Res 23, 1344–1352 (2016). https://doi.org/10.1007/s11356-015-5356-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5356-z