Abstract
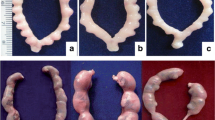
Estrogenic potential of Di-isobutyl phthalate (DIBP) and Di-isononyl phthalate (DINP) was studied using two different test systems. Two different doses of DIBP (250 and 1250 mg/kg) and DINP (276 and 1380 mg/kg) were administered to immature female rats (20 days old) orally once daily for 3 and 20 days in uterotrophic and pubertal assay, respectively. The animals were sacrificed on day 4 and day 41 in case of 3-day uterotrophic and 20-day pubertal assay, respectively. The results indicated that non-significant alterations in uterine and ovarian wet weight were observed in both the DIBP- and DINP-treated groups while the uterus weight increased significantly (i.e., 4–6 times) in the Diethylstilbesterol (DES)-treated group in both the assays. In the present study, precocious vaginal opening occurred at 26 days of age in the DES-treated group with a mean body weight of 30.39 ± 1.08 g. However, no precocious vaginal opening was found in any of the DIBP- and DINP-treated groups. The results indicated that both the phthalate compounds were unable to induce elevation in the uterine weight in both the assays and unable to cause vaginal opening indicating non-estrogenic potential of both the phthalate compounds, i.e., DIBP and DINP in vivo.



Similar content being viewed by others
References
Ahmad R, Verma Y, Gautam AK and Sunil Kumar (2013) Assessment of estrogenic potential of di-n-butyl phthalate and butyl benzyl phthalate in vivo. Toxicol Ind Health 1–8, doi:10.1177/0748233713491803.
ECPI (2006) DIBP Information Centre-CAS Number 84-69-5 [on line]. Available from URL: http://www.dibp-facts.com/ [Assesses 2006 Oct 30].
Erlandsson MC, Jonsson CA, Lindberg MK, Ohlsson C, Carlsten H (2002) Raloxifene- and estradiol-mediated effects on uterus, bone and B lymphocytes in mice. J Endocrinol 175:319–327
EU Risk Assessment Report for DEHP (2008) Final report, European Commission, EUR 23384EN, European Union Risk Assessment Report, Volume 80, Luxembourg: Office for Official Publications of the European Communities, ISSN1018-5593
Harris CA, Henttu P, Parker MG, Sumpter JP (1997) The estrogenic activity of phthalate esters in vitro. Environ Health Perspect 105:802–811
Kanno J, Onyon L, Haseman J, Fenner Crisp P, Ashby J, Owens W (2001) The OECD programme to validate the rat uterotrophic bioassay to screen compounds for in vivo estrogenic responses: phase 1. Environ Health Perspect 109:785–94
Kanno J, Onyon L, Haseman J, Fenner Crisp P, Ashby J, Owens W (2003) The OECD programme to validate the rat uterotrophic bioassay to screen compounds for in vivo estrogenic responses: phase 2-coded single dose studies. Environ Health Perspect 111:1550–1558
Kim YJ, Ryu JC (2006) Evaluation of estrogenic effects of phthalate analogues using in vitro and in vivo screening assays. Mol Cell Toxicol 2:106–113
Kim HS, Shin JH, Moon HJ, Kang H, Il KTS, Kim YI, Seok HJ, Pyo MY, Han YS (2002a) Comparative estrogenic effects of p-nonylphenol by 3-day uterotrophic assay and female pubertal onset assay. Reprod Toxicol 16:259–268
Kim HS, Shin JH, Moon HJ, Kim TS, Kang H, Il SJH, Kim IY, Park KL, Han SY (2002b) Evaluation of the 20-day pubertal female assay in Sprague–Dawley rats treated with DES, tomaxifen, testosterone and flutamide. Toxicol Sci 67:52–62
Marty MS, Crissmann JW, Carney EW (1999) Evaluation of the EDSTAC female pubertal assay in CD rats using 17β-estradiol, steroid biosynthesis inhibitors and thyroid inhibitor. Toxicol Sci 52:269–277
Moore MR (1998) Oncogenicity study in mice with di(isononyl)phthalate including ancillary hepatocellular proliferation and biochemical analyses. Covance Laboratories 144 Inc., Vienna, VA 22182. For Aristech Chemical Corporation, Pittsburgh, PA 15230. January 29, 1998. Covance 2598–105.
Moore NP (2000) The oestrogenic potential of the phthalate esters. Reprod Toxicol 14:183–192
O’Connor JC, Davis LG, Frame SR, Cook JC (2000) Evaluation of a Tier I screening battery for detecting endocrine-active compounds (EACs) using the positive controls testosterone, coumestrol, progesterone, and RU486. Toxicol Sci 54:338–54
Organization for economic cooperation and development (OECD) (2007) additional data supporting the test guideline on the uterotrophic bioassay in rodents. OECD Environmental Health and Safety Publication Series on Testing and Assessment No. 67
Saillenfait AM, Sabate JP, Gallissot F (2006) Developmental toxic effects of di-isobutyl phthalate, the methyl-branched analogue of di-n-butylphthalate administration by gavage to rats. Toxicol Lett 165:39–46
SCENIHR (2008) SCENIHR opinion on the safety of medical devices containing DEHP-plasticized PVC or other plasticizers on neonates and other groups possibly at risk, European Commission
Soto AM, Chung KL, Sonneschein C (1994) The pesticides endosulfan, toxaphene and dieldrin have estrogenic effects on human estrogen sensitive cells. Environ Health Perspect 102:380–383
Swan TL, Davis BJ (2003) Mechanism of phthalate ester toxicity in the female reproductive system. Environ Health Perspect 111(2):139–145
Tyler CR, Jobling S, Sumpter JP (1998) Endocrine disruption in wildlife: a critical review of the evidence. Critic Rev Toxicol 28:319
Younglai EV (2009) Pollutants, sedentarism, circadian rhythm and their effects on fertility. Reprod Biol Insights 2:47–52
Zacharewski TR, Meek MD, Clemons JH, Wu ZF, Fielden MR, Matthews JB (1998) Examination of the in vitro and in vivo estrogenic activities of eight commercial phthalate esters. Toxicol Sci 46:282–293
Zota AR, Calafat AM, Woodruff TJ (2014) Temporal trends in phthalate exposures: findings from the national health and nutrition examination survey, 2001–2010. Environ Health Perspect 122:235–241
Acknowledgments
Authors are thankful to the Indian Council of Medical Research (ICMR), New Delhi, for the financial assistance in the form of an ad hoc research project to one of us (SK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Sedha, S., Gautam, A.K., Verma, Y. et al. Determination of in vivo estrogenic potential of Di-isobutyl phthalate (DIBP) and Di-isononyl phthalate (DINP) in rats. Environ Sci Pollut Res 22, 18197–18202 (2015). https://doi.org/10.1007/s11356-015-5021-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5021-6