Abstract
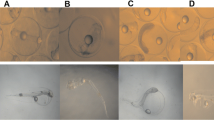
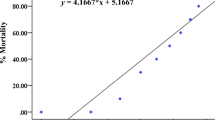
Rotenone, a natural compound derived from plants of the genera Derris and Lonchocarpus, is used worldwide as a pesticide and piscicide. This study aims to assess short-term toxicity of rotenone to early-life stages of the fish Danio rerio and Poecilia reticulata using a wide and integrative range of biomarkers (developmental, biochemical, behavioral, and histopathological). Moreover, the species sensitivity distribution (SSD) approach was used to compare rotenone acute toxicity to fish species. Toxicity tests were based on the OECD protocols, fish embryo toxicity test (for D. rerio embryos), and fish acute toxicity test (for P. reticulata juveniles). D. rerio embryos were used to estimate lethal concentrations and analyze embryonic and enzymatic alterations (activity of catalase, glutathione-S-transferase, and cholinesterase), while P. reticulata juveniles were used for the assessment of histological damage in the gills and liver. Rotenone induced significant mortality in zebrafish embryos with a 96-h lethal concentration 50 % (LC50) = 12.2 μg/L. Rotenone was embryotoxic, affecting the development of D. rerio embryos, which showed cardiac edema; tail deformities; loss of equilibrium; and a general delay characterized by lack of tail detachment, delayed somite formation, yolk sac absorption, and lack of pigmentation. Biochemical biomarker inhibition was observed for concentrations ≥1 μg/L for CAT and glutathione-S-transferase (GST) and for cholinesterase (ChE) in concentration from 10 μg/L. Behavioral changes were observed for P. reticulata juveniles exposed to concentrations equal to or above 25 μg/L of rotenone; moreover, histological damage in the liver and gills of fish exposed to concentrations equal to or above 2.5 μg/L could be observed. A hazard concentration 5 % (HC5) of 3.2 μg/L was estimated considering the acute toxicity data for different fish species (n = 49). Lethal and sublethal effects of rotenone raise a concern about its effects on nontarget fish species, especially because rotenone and its metabolite rotenolone are frequently reported in the microgram range in natural environments for several days after field applications. Rotenone should be used with caution. Given the high toxicity and wide range of sublethal effects here reported, further studies in a chronic exposure scenario are recommended.






Similar content being viewed by others
References
Ba-Omar TA, Al-Jardani S, Victor R (2011) Effects of pesticide temephos on the gills of Aphanius dispar (Pisces: Cyprinodontidae). Tissue Cell 43:29–38
Bernet D, Schmidt H, Meier W et al (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34
Betarbet R, Sherer TB, MacKenzie G et al (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306
Bills TD, Marking LL, Mauck WL (1981) Polychlorinated biphenyl (Aroclor 1254) residues in rainbow trout: effects on sensitivity to nine fishery chemicals. N Am J Fish Manag 1:200–203
Boogaard MA, Bills TD, Selgeby JH, Johnson DA (1996) Evaluation of piscicides for control of ruffe. N Am J Fish Manag 16:600–607
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bridges WR, Cope OB (1965) The relative toxicities of similar formulations of pyrethrum and rotenone to fish and immature stoneflies.
Broderius SJ, Kahl MD, Hoglund MD (1995) Use of joint toxic response to define the primary mode of toxic action for diverse industrial organic chemicals. Environ Toxicol Chem 14:1591–1605
Cengiz EI (2006) Gill and kidney histopathology in the freshwater fish Cyprinus carpio after acute exposure to deltamethrin. Environ Toxicol Pharmacol 22:200–204
Chadderton L, Kelleher S, Brow A et al (2001) Testing the efficacy of rotenone as a piscicide for New Zealand pest fish species. Manag. invasive Freshw. fish New Zealand. Proc. a Work. hosted by Dep. Conserv 10–12
Cheng WW, Farrell AP (2007) Acute and sublethal toxicities of rotenone in juvenile rainbow trout (Oncorhynchus mykiss): swimming performance and oxygen consumption. Arch Environ Contam Toxicol 52:388–396
Clairborne A (1985) Catalase activity. In: Greenwald RA (Ed.) CRC handbook of methods for oxygen radical research. Boca Raton, FL, USA. CRC Press, p 283–284
Clemens HP, Sneed KE (1959) Lethal doses of several commercial chemicals for fingerling channel catfish. US Department of the Interior, Fish and Wildlife Service
Cohen JM, Kamphake LJ, Lemke AE et al (1960) Effect of fish poisons on water supplies, part 1—removal of toxic materials. J Am Water Work Assoc 52:1551–1566
Crawford AD, Liekens S, Kamuhabwa AR et al (2011) Zebrafish bioassay-guided natural product discovery: isolation of angiogenesis inhibitors from East African medicinal plants. PLoS One 6:e14694
Cruz-Lacierda ER (1992) Toxicity of rotenone to milkfish, Chanos chanos, and tilapia, Oreochromis mossambicus. In: Shariff M, Subasinghe RP, Arthur JR (Eds). Diseases in Asian Aquaculture I. Proc First Symp Dis Asian Aquac. Manilla, Philippines. Asian Fisheries Society, pp 419–423
Da Cuna RH, Rey Vázquez G, Piol MN et al (2011) Assessment of the acute toxicity of the organochlorine pesticide endosulfan in Cichlasoma dimerus (Teleostei, Perciformes). Ecotoxicol Environ Saf 74:1065–1073
Ellman GL, Courtney KD, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fabacher DL, Chambers H (1972) Rotenone tolerance in mosquitofish. Environ Pollut 3:139–141
Ferguson HW (2006) Systemic pathology of fish. A text and atlas of normal tissues in teleosts and their responses in disease. Scotian Press, London
Finlayson BJ (2001) Introduction: Proceedings of the Symposium Rotenone in Fisheries. In: Cailteux et al. 2001, pp. 1–3. Available at: http://www.fisheries.org/units/rotenone/rewards/00intro.pdf[Internet
Finlayson BJ, Eilers JM, Huchko HA (2014) Fate and behavior of rotenone in Diamond Lake, Oregon, USA following invasive tui chub eradication. Environ Toxicol Chem 33:1650–1655
Frasco MF, Guilhermino L (2002) Effects of dimethoate and beta-naphthoflavone on selected biomarkers of Poecilia reticulata. Fish Physiol Biochem 26:149–156
Geiger DL, Brooke LT CD (1990) Acute toxicities of organic chemicals to fathead minnows (Pimephales promelas), Volume V. Center for Lake Superior Environment Studies, University of Wisconsin-Superior
Geiger D L, Poirier S H, Brooke L T CDJ (1986) Acute toxicities of organic chemicals to fathead minnows (Pimephales promelas), Volume III. Center for Lake Superior Environment Studies, University of Wisconsin-Superior
Giasson BI, Lee VM-Y (2000) A new link between pesticides and Parkinson’s. Nat Neurosci 3:1227
Gilderhus PA (1982) Effects of an aquatic plant and suspended clay on the activity of fish toxicants. N Am J Fish Manag 2:301–306
Gingerich WH, Rach JJ (1985) Uptake, biotransformation, and elimination of rotenone by bluegills (Lepomis macrochirus). Aquat Toxicol 6:179–196
Guadaño A, González-Coloma A, de la Peña E (1998) Genotoxicity of the insecticide rotenone in cultured human lymphocytes. Mutat Res Gen Toxicol Environ Mutagen 414(1):1–7
Haag HB (1931) Toxicological studies of Derris elliptica and its constituents I. Rotenone. J Pharmacol Exp Ther 43:193–208
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione transferases. Methods Enzymol 77:398–405
Hanisch K, Küster E, Altenburger R, Gündel U (2010) Proteomic signatures of the zebrafish (Danio rerio) embryo: sensitivity and specificity in toxicity assessment of chemicals. Int J Proteomics 2010:1–13
Hashimoto Y, Nishuichi Y (1981) Establishment of bioassay methods for evaluation of acute toxicity of pesticides to aquatic organisms. J Pestic Sci 6:257–264
Hinton MJ, Eversole AG (1978) Toxicity of ten commonly used chemicals to American eels. Proc Annu Conf Southeast Assoc Fish Wildl Agencies 32:599–604
Hinton MJ, Eversole AG (1979) Toxicity of ten chemicals commonly used in aquaculture to the black eel stage of the American eel. Proc World Maric Soc Wiley Online Library, pp 554–560
Holcombe GW, Phipps GL, Sulaiman AH, Hoffman AD (1987) Simultaneous multiple species testing: acute toxicity of 13 chemicals to 12 diverse freshwater amphibian, fish, and invertebrate families. Arch Environ Contam Toxicol 16:697–710
Howland RM (1969) Interaction of antimycin A and rotenone in fish bioassays. Prog Fish Cult 31:33–34
Hung MW, Zhang ZJ, Li S et al (2012) From omics to drug metabolism and high content screen of natural product in zebrafish: a new model for discovery of neuroactive compound. Evid Based Complement Altern Med 2012:1–20
Jesus FT, Oliveira R, Silva A et al (2013) Lethal and sub lethal effects of the biocide chlorhexidine on aquatic organisms. Ecotoxicology 22:1348–1358
Ling N (2003) Rotenone: a review of its toxicity and use for fisheries management. Sci Conserv 211:1–40
Marking LL, Bills TD (1976) Toxicity of rotenone to fish in standardized laboratory tests. US Fish. Wildlife Serv. Investigation in Fish Control 72:1–11
Marking LL, Bills TD (1981) Sensitivity of four species of carp to selected fish toxicants. N Am J Fish Manag 1:51–54
Marking LL, Bills TD, Crowther JR (1984) Effects of five diets on sensitivity of rainbow trout to eleven chemicals. Prog Fish Cult 46:1–5
Mascaro UCP, Rodrigues LA, Bastos JK, Santos E, Chaves da Costa JP (1998) Valores de DL50 em peixes e no rato tratados com pó de raízes de Derris spp e suas implicações ecotoxicológicas. Pesqui Vet Bras 18:53–56
Mayer FLJ (1974) Pesticides as pollutants. In: Liptak BG (ed) Environmental engineer’s handbook. Chilton Book Co., Radnor, PA, USA, pp 405–418
Mayer FL, Ellersieck MR (1986) Manual of acute toxicity: interpretation and data base for 410 chemicals and 66 species of freshwater animals. US Department of the Interior, Fish and Wildlife Service, Washington DC
Meadows BS (1973) Toxicity of rotenone to some species of coarse fish and invertebrates. J Fish Biol 5:155–163
Melo KM, Grisolia CK, Pieczarka JC et al (2014) FISH in micronucleus test demonstrates aneugenic action of rotenone in a common freshwater fish species, Nile tilapia (Oreochromis niloticus). Mutagenesis 29:215–219
Nanda NBP, Das PC, Jena J (2009) Use of rotenone as piscicide: toxicity levels in a few common freshwater predatory and weed fishes. J Appl Aquac 21:241–249
OECD (1992) Guideline for testing chemicals. Fish Acute Toxicity Test 203:1–9
OECD (2013) Test 236: Fish Embryo Acute Toxicity (FET) Test. OECD Guideline for the Testing of Chemicals,Section 2, OECD Publishing, Paris, France. doi:10.1787\9789264203709-en
Olson LE, Marking LL (1975) Toxicity of four toxicants to green eggs of salmonids. Prog Fish Cult 37:143–147
Orciari RD (1979) Rotenone resistance of golden shiners from a periodically reclaimed pond. Trans Am Fish Soc 108:641–645
Pereira SPP, Oliveira R, Coelho S et al (2014) From sub cellular to community level: toxicity of glutaraldehyde to several aquatic organisms. Sci Total Environ 470:147–158
Plhalová L, Mácová S, Dolezelová P et al (2010) Comparison of Terbutryn acute toxicity to Danio rerio and Poecilia reticulata. Acta Vet Brno 79:593–598
Posthuma L, Suter GW II, Traas TP (2001) Species sensitivity distributions in ecotoxicology. Lewis Publishers, Boca Raton, FL, USA
Radad K, Rausch WD, Gille G (2006) Rotenone induces cell death in primary dopaminergic culture by increasing ROS production and inhibiting mitochondrial respiration. Neurochem Int 49:379–386
Rao KV, Chauhan SPS (1971) Teratogenic effects of rotenone on the early development of chick embryos in vitro. Teratology 4:191–197
Rowe-Rowe DT (1971) Rotenone tolerances of some freshwater fishes of Natal. Prog Fish Cult 33:206–209
Siddiqui MA, Ahmad J, Farshori NN et al (2013) Rotenone-induced oxidative stress and apoptosis in human liver HepG2 cells. Mol Cell Biochem 384:59–69
Skadsen JM, Webb PM, Kostecki PT (1980) Measurement of sublethal metabolic stress in rainbow trout (Salmo gairdneri) using automated respirometry. J Environ Sci Health B 15:193–206
SPSS (2004) Sigma Stat for Windows (version 3.10).
Srivastava P, Panda D (2007) Rotenone inhibits mammalian cell proliferation by inhibiting microtubule assembly through tubulin binding. FEBS J 274:4788–4801
Swarnkar S, Goswami P, Kamat PK et al (2013) Rotenone-induced neurotoxicity in rat brain areas: a study on neuronal and neuronal supportive cells. Neuroscience 230:172–183
Turner L, Jacobson S, Shoemaker L (2007) Risk assessment for piscicidal formulations of rotenone. Compliance Services International, Lakewood
USEPA (2006) Enviromental fate and ecological risk assessment for the registration of rotenone. Prepared by Phillips T, Steeger T, Jones R D. Washington DC, 203. Environ Fate Eff Div U S EPA
USEPA (2014) Pesticide ecotoxicity database, formerly: environmental effects database (EEDB). Environ Fate Eff Div U S EPA
Vasquez ME, Rinderneck J, Newman J, McMillin S, Finlayson B, Mekebri A, Crane D, Tjeerdema S (2012) Rotenone formulation fate in Lake Davis following the 2007 treatment. Environ Toxicol Chem 31:1032–1041
Acknowledgments
The authors acknowledge the National Council of Technological and Scientific Development (CNPq), Coordination of Improvement of Higher Education Personnel (CAPES; scholarship provided to Karina Motta Melo), Ministry of Education, and Ministry of Science and Technology of Brazil through the program Science without Borders (CNPq; BJT-A scholarship provided to Rhaul Oliveira). The authors also acknowledge the Science Support Foundation of Para (FAPESPA) for financial support through the National Program of Research Excellence (PRONEX, 011/2008) and the Portuguese Fundação para Ciência e Tecnologia (FCT) for the financial support through the grants for the authors (SFRH/BPD/31752/2006, SFRH/BD/62605/2009). CYN, JCP, and CKG are grateful to CNPq for the Bolsas de Produtividade. This study is part of the Master’s Dissertation of KMM. In memoriam José de Souza Filho.
Ethical standards
The experiments are in accordance with the current laws of the country in which they were performed. The study was approved by the ethics committee, at the Federal University of Para (reference BIO036-12).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
Highlights
- Rotenone is extremely toxic for fish with a HC5 = 3.2 μg/L.
- Behavioral, structural, and developmental changes occur after exposure to low concentrations of rotenone.
- Biomarkers of oxidative (CAT and GST) and neurological (ChE) stress are inhibited by rotenone.
In memoriam of José de Souza Filho.
Rights and permissions
About this article
Cite this article
Melo, K.M., Oliveira, R., Grisolia, C.K. et al. Short-term exposure to low doses of rotenone induces developmental, biochemical, behavioral, and histological changes in fish. Environ Sci Pollut Res 22, 13926–13938 (2015). https://doi.org/10.1007/s11356-015-4596-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4596-2