Abstract
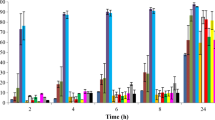
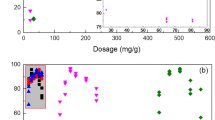
This study aimed to investigate the performance of chitosan-modified nano-sized montmorillonite (CTS/NMMT) during the flocculation of Microcystis aeruginosa (MA). The release of intracellular microcystins (MCs) caused by the damage of intact MA cells during the flocculation and floc storage processes was also comprehensively evaluated through scanning electron microscopy (SEM) and measurement of K+ and Mg2+ release. With the application of the Box–Behnken experimental design combined with response surface methodology, the quadratic statistical model was established to predict and optimize the interactive effects of content of CTS/NMMT, weight ratio of NMMT to CTS, and agitation time on the removal efficiency of MA cells. A maximum removal of 94.7 % MA cells was observed with content of CTS/NMMT 300–320 mg L−1, weight ratio of NMMT to CTS 14–16, and agitation time 16–50 min. During the flocculation process, CTS/NMMT aggregated MA cells as flocs and served as a protection shield for cells. The extracellular and intracellular microcystin–leucine–arginine (MC-LR) decreased remarkably and the yield of intracellular MC-LR showed a decreasing trend during the flocculation. The cell integrity was slightly damaged by the mechanical actions rather than by the flocculant. During the floc storage process, cell lysis and membrane damage were remarkably aggravated. The noticeable increase of K+ and Mg2+ release indicated that CTS/NMMT damaged the integrity of most MA cells in the flocs and liberated the intracellular MC-LR. Meanwhile, NMMT and CTS polymers assisted the adsorptive removal of extracellular MC-LR released to water. The flocs should be timely treated within 12 h to prevent the leakage of MCs.








Similar content being viewed by others
References
Auta M, Hameed B (2014) Chitosan–clay composite as highly effective and low-cost adsorbent for batch and fixed-bed adsorption of methylene blue. Chem Eng J 237:352–361
Campos A, Vasconcelos V (2010) Molecular mechanisms of microcystin toxicity in animal cells. Int J Mol Sci 11:268–287
Carmichael WW (2001) Health effects of toxin-producing cyanobacteria:“The CyanoHABs”. Hum Ecol Risk Assess 7:1393–1407
Chen C-Y, Chung Y-C (2011) Comparison of acid-soluble and water-soluble chitosan as coagulants in removing bentonite suspensions. Water Air Soil Pollut 217:603–610
Dong C, Chen W, Liu C (2014) Flocculation of algal cells by amphoteric chitosan-based flocculant. Bioresour Technol 170:239–247
Drikas M, Chow CW, House J, Burch MD (2001) Using coagulation, flocculation, and settling to remote toxic cyanobacteria. J Am Water Works Assoc 93:100–111
Farid MS, Shariati A, Badakhshan A, Anvaripour B (2013) Using nano-chitosan for harvesting microalga Nannochloropsis sp. Bioresour Technol 131:555–559
Ho L et al (2012) Fate of cyanobacteria and their metabolites during water treatment sludge management processes. Sci Total Environ 424:232–238
Hu X et al (2014) Effects of d-menthol stress on the growth of and microcystin release by the freshwater cyanobacterium Microcystis aeruginosa FACHB-905. Chemosphere 113:30–35
Hua Z, Gang P, Hao C, Xianzheng Y (2006) Removal of cyanobacterial blooms in Taihu Lake using local soils II. Effective removal of Microcystis aeruginosa using local soils and sediments modified by chitosan. Environ Pollut 141(2):201–205
Imandi SB, Bandaru VR, Somalanka SR, Garapati HR (2007) Optimization of medium constituents for the production of citric acid from byproduct glycerol using Doehlert experimental design. Enzym Microb Technol 40:1367–1372
Jančula D, Maršálek B (2011) Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 85:1415–1422
Kumar MS, Phanikumar B (2013) Response surface modelling of Cr6+ adsorption from aqueous solution by neem bark powder: Box–Behnken experimental approach. Environ Sci Pollut Res 20:1327–1343
Li X et al (2015) The fate of Microcystis aeruginosa cells during the ferric chloride coagulation and flocs storage processes. Environ Technol 36(7):920–928
Liu H, Du Y, Wang X, Sun L (2004a) Chitosan kills bacteria through cell membrane damage. Int J Food Microbiol 95:147–155
Liu HL, Lan YW, Cheng YC (2004b) Optimal production of sulphuric acid by Thiobacillus thiooxidans using response surface methodology. Process Biochem 39:1953–1961. doi:10.1016/j.procbio.2003.09.018
Long BM, Jones GJ, Orr PT (2001) Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl Environ Microbiol 67:278–283
Ma M, Liu R, Liu H, Qu J (2012) Chlorination of Microcystis aeruginosa suspension: cell lysis, toxin release and degradation. J Hazard Mater 217:279–285
Pan G, Zhang M-M, Chen H, Zou H, Yan H (2006a) Removal of cyanobacterial blooms in Taihu Lake using local soils. I. Equilibrium and kinetic screening on the flocculation of Microcystis aeruginosa using commercially available clays and minerals. Environ Pollut 141:195–200
Pan G, Zou H, Chen H, Yuan X (2006b) Removal of harmful cyanobacterial blooms in Taihu Lake using local soils III. Factors affecting the removal efficiency and an in situ field experiment using chitosan-modified local soils. Environ Pollut 141:206–212
Pei H-Y, Ma C-X, Hu W-R, Sun F (2014) The behaviors of Microcystis aeruginosa cells and extracellular microcystins during chitosan flocculation and flocs storage processes. Bioresour Technol 151:314–322
Polyak Y, Zaytseva T, Medvedeva N (2013) Response of toxic cyanobacterium Microcystis aeruginosa to environmental pollution. Water Air Soil Pollut 224:1–14
Renault F, Sancey B, Badot P-M, Crini G (2009) Chitosan for coagulation/flocculation processes—an eco-friendly approach. Eur Polym J 45:1337–1348
Shao J et al (2012) Physiological responses of Microcystis aeruginosa NIES-843 (cyanobacterium) under the stress of chitosan modified kaolinite (CMK) loading. Ecotoxicology 21:698–704
Sun F, Pei H-Y, Hu W-R, Ma C-X (2012) The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes. Chem Eng J 193:196–202
Sun F, Pei H-Y, Hu W-R, Li X-Q, Ma C-X, Pei R-T (2013) The cell damage of Microcystis aeruginosa in PACl coagulation and floc storage processes. Sep Purif Technol 115:123–128
Thirumavalavan M, Hu Y-L, Lee J-F (2012) Effects of humic acid and suspended soils on adsorption and photo-degradation of microcystin-LR onto samples from Taiwan reservoirs and rivers. J Hazard Mater 217:323–329
Venrick E (1978) How many cells to count. Phytoplankton manual. UNESCO, Paris, pp 167–180
Wan Ngah W, Teong L, Hanafiah M (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83:1446–1456
Wang L, Wang A (2007) Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. J Hazard Mater 147:979–985
Wang ZY, Wang C, Wang PF, Qian J, Hou J, Ao YH (2014) Process optimization for microcystin-LR adsorption onto nano-sized montmorillonite K10: application of response surface methodology. Water Air Soil Pollut 225(9):1–18. doi:10.1007/S11270-014-2124-5
Xu H, Cai H, Yu G, Jiang H (2013) Insights into extracellular polymeric substances of cyanobacterium Microcystis aeruginosa using fractionation procedure and parallel factor analysis. Water Res 47:2005–2014
Yan H, Gong A, He H, Zhou J, Wei Y, Lv L (2006) Adsorption of microcystins by carbon nanotubes. Chemosphere 62:142–148
Yetilmezsoy K, Saral A (2007) Stochastic modeling approaches based on neural network and linear-nonlinear regression techniques for the determination of single droplet collection efficiency of countercurrent spray towers. Environ Model Assess 12:13–26. doi:10.1007/s10666-006-9048-4
Zeng D, Wu J, Kennedy JF (2008) Application of a chitosan flocculant to water treatment. Carbohydr Polym 71:135–139
Zhang YQ, Wu QP, Zhang JM, Yang XH (2011) Effects of ozone on membrane permeability and ultrastructure in Pseudomonas aeruginosa. J Appl Microbiol 111:1006–1015. doi:10.1111/j.1365-2672.2011.05113.x
Zhang Z, Pang Q, Li M, Zheng H, Chen H, Chen K (2015) Optimization of the condition for adsorption of gallic acid by Aspergillus oryzae mycelia using Box-Behnken design. Environ Sci Pollut Res 22:1085–1094
Zhou S, Shao Y, Gao N, Deng Y, Qiao J, Ou H, Deng J (2013) Effects of different algaecides on the photosynthetic capacity, cell integrity and microcystin-LR release of Microcystis aeruginosa. Sci Total Environ 463–464:111–119. doi:10.1016/j.scitotenv.2013.05.064
Acknowledgments
We are grateful to all anonymous editors and reviewers for providing comments on this manuscript. We also appreciate the generous financial support of this work provided by the National Science Fund for Creative Research Groups of China (No. 51421006), the National Science Fund for Distinguished Young Scholars (No. 51225901), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13061), the Outstanding Youth Fund of Jiangsu Province (No. BK2012037), the Fundamental Research Fund for the Central Universities (No. 2014B03814), and PAPD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Wang, Z., Wang, C., Wang, P. et al. The performance of chitosan/montmorillonite nanocomposite during the flocculation and floc storage processes of Microcystis aeruginosa cells. Environ Sci Pollut Res 22, 11148–11161 (2015). https://doi.org/10.1007/s11356-015-4412-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4412-z