Abstract
Intensive lignite and glass sand mining and industrial processing release waste which may contain elements hazardous to the aquatic ecosystem and constitute a potential risk to human health. Therefore, their levels must be carefully controlled. As a result, we examined the effects of sewage on the aquatic Fontinalis antipyretica moss in the Nysa Łużycka (lignite industry) and the Kwisa Rivers (glass sand industry). The Nysa Łużycka and the Kwisa Rivers appeared to be heavily polluted with As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, V and Zn, which were reflected in the extremely high concentration of these elements in F. antipyretica along the studied watercourses. In the Nysa Łużycka, trace element composition in the moss species is affected by lignite industry with accumulation in its tissues of the highest concentrations of Cd, Co, Cr, Cu, Mn, Ni, Pb and Zn, while samples from the Kwisa sites influenced by glass sand industry revealed the highest concentrations of As, V and Fe. The principal component and classification analysis classifies the concentration of elements in the aquatic F. antipyretica moss, thus enabling the differentiation of sources of water pollution in areas affected by mining industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The metals often present in industrial wastewaters are hazardous to the aquatic ecosystem and may affect the quality of life. Therefore, their elimination from the aquatic environment plays an important role in water pollution control. As observed by Markert et al. (2003) and Vázquez et al. (2004), pollution monitoring using bioaccumulators is one of the methods for the evaluation of xenobiotic levels. Plants accumulate trace elements and provide an indication of their soluble fraction in the surrounding environment which is likely to affect major compartments of the aquatic ecosystem (Rasmussen and Andersen 1999; Kłos et al. 2010; Krems et al. 2013). Owing to these “accumulating bioindicators”, the level of pollutants in the environment can be studied (Remon et al. 2013). Aquatic mosses are commonly considered invaluable, efficient accumulators of pollutants and ideal indicators of metal contamination (de Traubenberg and Ah-Peng 2004; Pekka et al. 2008; Vuori and Helisten 2010; Vázquez et al. 2013; Cesa et al. 2014). Due to their morphological and physiological characteristics, their ability to accumulate xenobiotics and their widespread occurrence, aquatic mosses are very useful in bioindication and biogeochemical prospecting (Bleuela et al. 2005). Aquatic mosses have a greater accumulation capacity for metals than sediment or vascular macrophytes and allow the characterisation of contaminant bioavailability (Fernández et al. 2006; Dazy et al. 2009). These plants gauge the quality of the environment providing an integrated assessment of a typically discontinuous series of contamination events (Siebert et al. 1996). One of the most commonly used taxa for biomonitoring is Fontinalis antipyretica Hedw. (Fontinalaceae), a moss widely distributed in the northern hemisphere and shown to accumulate different contaminants at high levels (Bleuela et al. 2005; Vuori and Helisten 2010; Díaz et al. 2013). This species lacks a well-developed cuticle and vascular tissue, which enables metal uptake directly from water by adsorption and absorption through cell surfaces (Davies 2007; Vuori and Helisten 2010). F. antipyretica has a capacity to characterise the quality of sewage-affected water, which may give an indication of the level of ecological risk due to pollution (Vázquez et al. 2013).
The biomass of this aquatic moss can be used as a sorbent for the purification of metal-bearing waste water (Martins et al. 2004; Rau et al. 2007). Many natural rivers have been exposed to metal contamination from anthropogenic sources. In this investigation, two rivers were selected: the Nysa Łużycka polluted with Bogatynia lignite industry sewage and the Kwisa receiving effluents from the Osiecznica glass sands mine. Lignite mining industry is a major source of trace elements and other pollutants influencing ecosystem development (Maiti 2007). The Osiecznica glass sand deposit is enriched with heavy minerals which may be a source of considerable environmental pollution (Łuszczkiewicz 1987). The aim of this study was to investigate the level of contaminants (As, Cd, Co, Cr Cu, Fe, Mn, Ni, Pb, V and Zn) in F. antipyretica collected from the two rivers affected by different types of pollution. These results were juxtaposed with similar analyses of water samples collected from the same sites, which allowed us to determine the elemental composition of the water both directly and indirectly, using a bioindicator organism (Vázquez et al. 2004). The tested hypotheses were (1) whether the ubiquitous F. antipyretica may be used as a suitable bioindicator of toxic elements in sewage from glass sand processing and mining and from lignite industry and (2) whether principal component and classification analysis classifying the concentration of elements in F. antipyretica would enable differentiation of the origin of pollution through relevant patterns.
Materials and methods
Two rivers in the Lower Silesia (SW Poland) were selected in which F. antipyretica occurred naturally (Fig. 1). The Nysa Łużycka (198 km in length) is the second largest left tributary of the Oder. The river flows into Poland from the Czech Republic through the Zittau-Zgorzelec Basin between the Izera Foreland in Poland and the Lusatian Foreland in Germany. Particular characteristic is a narrow gorge of Precambrian granitoids and Neogene basalts. The Nysa Łużycka flows into the Silesian Lowland upstream from Zgorzelec. In the west, the Nysa Łużycka valley is delimited by the Lower Silesian Forest. The main lithology of the Nysa Łużycka consists of fluvial deposits (sands, gravels, muds, peats and organic silts) (Badura and Przybylski 2000; Marks et al. 2006; Badura et al. 2012). The depth of the river is highly variable and ranges from several dozen of centimetres to several metres in the investigated area. River width in the section concerned is approximately 5–10 m. The river is classified in terms of its hydrological regime as a mountain and piedmont river, with flow rate ranging from 0.03 to 16.22 m3/s. The catchment area of the Nysa Łużycka is subject to a strong human impact associated with mining activities and intensification of water economy. Thus, the natural water balance is significantly disturbed (Badura and Przybylski 2000). The Kwisa River (140 km in length) is the largest tributary of the Bóbr River. The source of the river lies at an altitude of approximately 900 m above the sea level in the Izera Mountains. The Kwisa flows in the study area through a vast complex of the Lower Silesian Forest. The bottom of the meandering valley comprises typical fluvial deposits (sands, gravels, muds, peats and organic silts). Outcrops of the Upper Cretaceous sandstones, marls and mudstones occur in the southern part of the river (the Kaczawa foreland) (Badura and Przybylski 2000; Marks et al. 2006; Badura et al. 2012). This river has an average width of approximately 15 m, the depth ranges from 0.5 to 1 m in straight sections and 2.5 m in bends with average flow rate ranging from 1.01 to 7.28 m3/s (Badura and Przybylski 2000; Machajski 2012). In the case of the two rivers, the climate is temperate with humid air masses from the Atlantic (Najbar et al., 1999). The Nysa Łużycka receives sewage from Bogatynia lignite industry while the Kwisa is polluted by the Osiecznica glass sands mine. In each river, sampling sites were selected at regular intervals starting downstream from the outlet (sites 1–24 spaced 0.83 km from each other) and directly upstream from the sewage outlet (sites 25–30 spaced 2.5 km from each other). Samples of water and F. antipyretica were collected from either river on the same day. At each sampling point, three subsamples were taken along a line perpendicular to the river: one subsample in the middle of the river and two subsamples at both sides at a distance from the middle of 1/4 of stream width. Each replicate consisted of a tuft of about 20 g of wet moss. The mosses were washed thoroughly in river water to remove attached particles and epifauna. Basal stems were removed, and apices were cut to a length of 2 cm according to Vázquez et al. (2004). The total number of water and plant samples per river was 30 × 3 = 90.
Water and plant analysis
Prior to the analysis, the water samples were acidified to pH ≤ 2 with spectrally pure HNO3 and filtered through 0.45 μm glass microfiber filters to determine total As and metal concentrations (Ladislas et al. 2012). Three replicates were analysed separately.
The moss samples were dried at 50 °C to constant weight and homogenised to fine powder in an IKA Labortechnik M20 laboratory mill. Dried plant samples (300 mg dry weight, in triplicate) were digested with 3 mL of nitric acid (ultra pure, 65 %) and 2 mL of perchloric acid (ultra pure, 70 %) in a CEM Mars 5 microwave oven. The digests were then diluted with deionised water to a total volume of 50 mL and analysed together with the filtered water samples for Fe, Mn and Zn using FAAS and As, Cd, Co, Cr, Cu, Ni, Pb and V using ETAAS with a GF3000 Graphite Furnace (AVANTA PM Atomic Absorption Spectrophotometer from GBC Scientific Equipment). All elements were assayed against the Atomic Absorption Standard Solution from Sigma Chemical Co. and blanks containing the same matrix as the samples. Results of element concentrations in the plants were calculated on a dry weight basis. The concentrations of As, Co, Cu, Cr, Ni and V in water were below the detection limits (μg L−1) of 1.5, 0.1, 0.1, 0.05, 0.5 and 1.5, respectively. The accuracy of the methods applied for the determination of element concentrations in plant samples was verified against Certified Reference Materials: moss M2 and M3 (Finnish Forest Research Institute). A coefficient of variance (CV) was calculated for the measured metal concentrations in the reference materials (Table 1).
Statistical analysis
Differences between particular sampling sites in terms of element concentrations in water and mosses were evaluated by one-way ANOVA. The normality of the analysed features was verified using Shapiro-Wilk’s W test, and the homogeneity of variances was verified using the Brown-Forsythe test (Brown and Forsythe 1974; Argaç 2004). To obtain normal distribution of the features, data have been transformed with Box-Cox according to Zar (1999). Pearson correlations were calculated between the concentrations of elements in water and in moss tissues.
The matrix of concentrations of 11 elements (As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, V and Zn) in the moss samples collected from 48 sites downstream from the outlet in both rivers was subjected to numerical classification to identify groups of samples with similar patterns of metals. The clustering algorithm was prepared with complete linkage. The City-block (Manhattan) distance method was used for a similarity measure. Student’s t test was used to compare the concentration of trace elements between F. antipyretica groups as distinguished by cluster analysis.
The matrix of concentrations of 11 elements (As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, V and Zn) in the moss samples collected from 48 sites downstream from the outlet in both rivers was subjected to ordination to reveal possible gradients of concentration levels using principal component and classification analysis (PCCA). Plots of PCCA ordination of the plant samples and projection of element concentrations on the factor plane reveal similarities between the samples and correlations between the original variables and the first two factors (Legendre and Legendre 1998). The basis for PCCA is the principal component analysis often used in ecology to reduce the amount of data and stabilise subsequent statistical analyses (Vaughan and Ormerod 2005).
The contamination factor (CF) (Mouvet et al. 1986) was calculated as the ratio of metal concentration in the moss tested to the background level in that species established for the study area (the source area of the Nysa Łużycka river as investigated by Vázquez et al. (2004)). All calculations were done with Statistica 10 software (Statsoft. 2011).
Results
The ranges of As and metal concentrations in water and moss samples are presented in Tables 2 and 3. The mean concentrations of elements in water and mosses differed significantly (ANOVA, P < 0.05).
The pH of streamwater ranged between 6.6 and 7.5 for the Nysa Łużycka and between 6.8 and 7.6 for the Kwisa, with the lowest results observed in the vicinity of sewage outputs (sites 1–3 and 13–15 for the Nysa Łużycka and sites 1–3 for the Kwisa). The pH is an important factor which influences trace element bioavailability by affecting speciation and properties of biological surfaces (Lithner et al. 1995). The authors also report that at pH < 6 (i.e. lower than in the rivers tested), the bioaccumulation factor in F. antipyretica decreases for Cd, Co, Ni and Zn.
The investigated rivers, both upstream and downstream from the sewage outputs, exceed the permitted values of trace element content in unpolluted surface water as determined by Kabata-Pendias (2001) (Cd < 0.005, Fe <0.5, Mn < 0.1 and Pb < 0.05 mg L−1). Elevated levels of these contaminants were observed also in the water samples collected upstream from the sewage outputs in both rivers, which can be attributed to an anthropogenic influence in the investigated areas (Vázquez et al. 2004). The concentrations of the elements downstream from the sewage outputs were significantly higher (t test, P < 0.05) than in the upstream samples for Cd, Fe and Mn in the Nysa Łużycka and for Fe, Mn and Pb in the Kwisa. The downstream part of the Nysa Łużycka contained significantly higher Cd concentrations and lower Fe and Mn concentrations than the respective section of the Kwisa (Table 2).
A comparison of trace element concentrations in F. antipyretica collected from upstream to downstream sites (t test, P < 0.05) revealed that the respective concentrations of As, Cd, Cr, Ni, Pb, V and Zn (the Nysa Łużycka) as well as As, Co, Cu, Fe, Pb and V (the Kwisa) were considerably higher (t test, P < 0.05) in the mosses downstream from the sewage outputs (Table 3) and significantly exceeded the concentrations (in parentheses, mg kg−1) of Co (3.6), Cr (4.1), Cu (10), Fe (3763), Mn (849), Ni (8.8), Pb (7.4), V (0.6) and Zn (130) against the background values given by Vázquez et al. (2004) for F. antipyretica collected from the source area of the Nysa Łużycka.
The calculation of the bioconcentration factor (BCF) from the metal content in the moss and the concentration in water leads to the following conclusions in terms of the accumulation properties of the plant investigated (Siebert et al. 1996). The ranges of BCFs for F. antipyretica for Cd, Fe, Mn Pb and Zn from the Nysa Łużycka are the following: 12–294, 161–371, 1342–3152, 36–331 and 212–1170, respectively, and from the Kwisa: 20–1484, 164–764, 276–2364, 40–207 and 103–931, respectively. These BCFs were lower than the values established by Martins and Boaventura (2002) for Cu (31,400) and Zn (4531) in the same species. The reason may be that the metal uptake rate tends to decrease with increasing metal exposure in water, which implies a toxic effect in mosses and subsequent deterioration of their physiological status (Martins and Boaventura 2002; Díaz et al. 2012).
A dendrogram of the samples based on the concentration of the elements in F. antipyretica (Fig. 2) reveals similarities between particular sites. Two main clusters are formed: A (a grouping of Nysa Łużycka sites 4–12, 16–24 and all Kwisa sites) and B (a grouping of Nysa Łużycka sites 1–3 and 13–15). Cluster A is subdivided into two subclusters: A1 (Nysa Łużycka sites 16–24 and Kwisa sites 1–6) and A2 (Nysa Łużycka sites 4–12 and Kwisa sites 7–24); cluster B is also subdivided into two subclusters: B1 (Nysa Łużycka sites 13–15) and B2 (Nysa Łużycka sites 1–3). Subcluster A1 is further subdivided into two sub-subclusters: A1a (Kwisa sites 1–6) and A1b (Nysa Łużycka sites 16–24). Subcluster A2 is subdivided into two sub-subclusters: A2a (Kwisa sites 7–15) and A2b (Nysa Łużycka sites 4–12 and Kwisa sites 16–24). F. antipyretica collected from the sites in cluster A had a significantly lower concentration of As, Cd, Co, Cr, Cu, Mn, Ni and Zn (t test, P < 0.001) compared to plants from cluster B. This results from the fact that cluster B groups mosses collected from sites situated in the closest vicinity to the sewage outputs in the Nysa Łużycka. F. antipyretica included in cluster B1, affected by lignite industry sewage, had significantly higher concentrations of As, Cd, Co, Cr and Ni (t test, P < 0.001) than plants from cluster B2, which in turn contained significantly higher concentrations of Cu, Mn and Zn. Plants from cluster B2 grew in the mouth of the stream alimented by municipal sewage. Cu, Mn and Zn are typical components of roadside dust composed of gasoline exhausts, car components, oil lubricants and industrial and incinerator emissions (Wei and Yang 2010; Apeagyei et al. 2011; Nazzal et al. 2013). Additionally, fuel exhausts contain Mn which is often added as a combustion enhancer, smoke suppressant and to increase the octane rating (Lytle et al. 1994; Valavanidis et al. 2006).
Figure 3 presents PCCA ordination indicating groups distinguished by the Joining tree. The first principal component discriminated between F. antipyretica from the Nysa Łużycka (sites 1–3, group B2 in the dendrogram; sites 13–15, group B1 in the dendrogram; and sites 16–24, group A1b in the dendrogram) and the Kwisa (sites 1–6, group A1a in the dendrogram) for which factor 1 returned negative scores, and sites 7–15 of the Kwisa (group A2a in the dendrogram), sites 4–12 of the Nysa Łużycka and 16–24 of the Kwisa (group A2b in the dendrogram) where factor 1 returned positive scores. Sites 1–3 were located in the closest vicinity of the sewage output, while sites 13–15 of the Nysa Łużycka were located in an area where the river was alimented by a stream polluted with municipal and industrial sewage. Additionally, F. antipyretica from the Kwisa (sites 1–6) situated nearest to the sewage output had negative scores of factor 2. The projection of the variables on the factor plane showed that F. antipyretica from sites 1–3 and 13–15 of the Nysa Łużycka had the highest tissue concentrations of Cd, Co, Cr, Cu, Mn, Ni, Pb and Zn. The moss from the Kwisa collected next to the sewage output had the highest concentrations of As, V and Fe, while F. antipyretica from sites 7–15 and 16–24 of the Kwisa and sites 4–12 of the Nysa Łużycka had the lowest concentrations of the elements. The latter sites were situated further downstream from the sewage output where the respective metal concentrations were already diminished in the course of the transport as a result of many processes such as dilution by ground and surface waters, mineral precipitation or sorption of elements onto precipitates and the streambed (Palmer et al. 2011).
Ordination plot of F. antipyretica based on concentrations of the 11 elements: As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, V and Zn and projection of As and metal concentrations on the component plane. Symbols refer to the clusters, subclusters and sub-subclusters of Fig. 2
Discussion
Significant positive Pearson correlation was observed between the concentrations of Cd, Fe and Zn (P < 0.05) in the water and moss samples, while no such correlation was noted for Mn and Pb. An investigation by Say and Whitton (1983) reported the existence of significant positive correlations also between Mn and Zn concentrations in water and F. antipyretica. Siebert et al. (1996) calculated such relations between Cd and Zn concentrations in water and the species discussed here. However, Vázquez et al. (2004) observed no significant correlations between concentrations in water and in F. antipyretica with respect to most of the analysed elements. According to these authors, the bioavailability of metals to plants depends on a number of environmental factors and concentrations in water that usually show temporal variability. Mosses accumulate large quantities of metals in their cells during the period of high bioavailability in the environment. Once pollution has ceased and the environment is restored or cleaned up, the mosses start to release the previously accumulated metals, though at a slower rate compared to the accumulation rate during the phase of pollution (López et al. 1994; Vázquez et al. 2004). Metals concentrated in mosses are thought to reflect past metal levels in the environment (Yoshimura et al. 2000). Lithner et al. (1995) and Siebert et al. (1996) stated that correlations between certain metals in water and in F. antipyretica may lead to conclusive observations about the accumulation properties of this species.
According to Rasmussen and Andersen (1999), F. antipyretica is able to accumulate higher concentrations of Cu than of Cr. Copper is known to form complex bonds with organic materials and may thus more strongly bind with the moss than Cr (Rasmussen and Andersen 1999). In the present investigation, F. antipyretica was observed to accumulate more Cu than Cr only in the most polluted sites 1–3 of the Nysa Łużycka. These discrepancies may be caused by the fact that Rasmussen and Andersen’s investigation (1999) was carried out in brackish water. Martins et al. (2004) believe that F. antipyretica takes up much lower amounts of Zn than Cu. In the present study, the concentration of Cu was up to 32 times lower than that of Zn in the moss. Similar results, i.e. concentrations of Zn higher than of Cu in moss, were reported for F. antipyretica by Samecka-Cymerman et al. (2005). F. antipyretica in the examined streams yielded the highest BCF for Mn and Zn (up to 3152 and 931, respectively).
The trace element concentrations in F. antipyretica were used to determine the level of contamination in the examined rivers using the contamination factor (CF) (Mouvet et al. 1986; Vázquez et al. 2004). The CF for the upstream parts of both rivers showed clean sites (CF up to two times the background level or lower) for Pb, suspected pollution (2 < CF < 6) for Cu, Fe and Zn and moderate pollution (6 < CF < 18) for Co, Cr, Mn, Ni and V (Mouvet et al. 1986). The CF for downstream parts of both rivers revealed (Fig. 4) no clean sites. Extreme Co contamination (CF > 54) was detected in sites 1–3 and 13–15 of the Nysa Łużycka (Fig. 4), while all the other sites in this river can be classified with respect to this metal as severely polluted (18 < CF < 54) (Mouvet et al. 1986). For Mn, Ni, Pb and V, sites 1–3 in the Nysa Łużycka were severely contaminated (Fig. 4). The CF of Mn, Ni, Pb and V (sites 4–24, 13–21, 13–21, 4–6 and 12–15, respectively, in the same river) indicates severe pollution. Sites 1–3 in the Kwisa show severe contamination with Cr and Pb. Sites 1–24 can be classified as severely polluted with Co, while sites 1–15 as severely polluted with V. The calculated CF values indicated that both rivers were severely polluted with the metals mentioned above. The elevated element concentrations, as found in F. antipyretica, especially with respect to sites 1–3 in both rivers, i.e. those situated in the nearest vicinity of the sewage outputs, as well as site 13 and further downstream of the Nysa Łużycka, i.e. those situated at the mouth of the Witka stream, reflected the element composition of sewage produced by lignite, urban and glass sand industry. Our results are supported by the PCCA ordination (Fig. 3), which distinguished the F. antipyretica from the sites polluted with lignite industry sewage from that collected at the sites polluted with glass sand industry sewage. Elevated Cd, Co, Cr, Cu, Mn, Ni, Pb and Zn levels in samples of this species influenced by lignite industry are confirmed by Sarris et al. (2009), Suchara et al. (2011) and Jasion et al. (2013), all of whom reported these metals to be significantly accumulated in coal fly ashes emitted during lignite combustion. Elevated As, V and Fe levels in F. antipyretica influenced by glass sand industry sewage are in accordance with reports of Łuszczkiewicz (1987) and Muszer and Łuszczkiewicz (2006) on the Osiecznica deposit enriched with heavy minerals, including ilmenite, arsenopyrite, magnetite, hematite and pyrite. The Osiecznica deposits contain abundant ore minerals (simple sulphides which may be a source of undesired elements in products and intermediates).
Contamination factors, CF (calculated as the ratio of metal concentration in F. antipyretica to the background level in that species) for metals in sites from the Nysa Łużycka (a–c) and the Kwisa (d, e); contamination classes by Mouvet et al. (1986): no contamination, CF < 2; suspected contamination, 2 < CF < 6; moderate contamination, 6 < CF < 18; severe contamination, 18 < CF < 54; extreme contamination, CF > 54
This investigation contributes to a better understanding of the relation between the concentrations of elements in F. antipyretica and in water in an effort to evaluate the potential pollution in a river. The use of this particular species is very important in bioindication as the pattern of variability of elements in F. antipyretica can be used to confirm the type of pollution.
Conclusion
-
(1)
The Nysa Łużycka and the Kwisa rivers were heavily polluted with As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, V and Zn, which were reflected in the extremely high concentration of these elements in the locally growing F. antipyretica.
-
(2)
F. antipyretica from the river receiving lignite industry sewage accumulated the highest concentrations of Cd, Co, Cr, Cu, Mn, Ni, Pb and Zn in its tissues, while that from the river influenced by glass sand industry sewage accumulated the highest concentrations of As, V and Fe.
-
(3)
The principal component and classification analysis classifies the concentration of elements in the aquatic moss F. antipyretica, which enables differentiation between sources of pollution in the rivers in particular areas of mining industry.
-
(4)
The results of this investigation permit conclusions as to the water pollution level in both rivers on the basis of the concentrations of trace elements observed in F. antipyretica.
References
Apeagyei E, Bank MS, Spengler JD (2011) Distribution of heavy metals in road dust along an urban–rural gradient in Massachusetts. Atmos Environ 45:2310–2323
Argaç D (2004) Testing for homogeneity in a general one-way classification with fixed effects: power simulations and comparative study. Comput Stat Data An 44:603–612
Badura J, Przybylski B (2000) Morphologic and age correlation of terraces of main rivers in the Lower Silesia. Państwowy Instytut Geologiczny, Warszawa
Badura J, Cymerman Z, Kozdrój W (2012) Szczegółowa mapa geologiczna polski. Arkusz Niesky (718), Węgliniec (719) [Detailed geological map of Poland. Sheet Niesky (718), Węgliniec (719)]. Państwowy Instytut Geologiczny, Warszawa [in polish]
Bleuela C, Wesenberg D, Sutter K, Miersch J, Braha B, Bärlocher F, Krauss GJ (2005) The use of the aquatic moss Fontinalis antipyretica L. ex Hedw. as a bioindicator for heavy metals 3. Cd2+ accumulation capacities and biochemical stress response of two Fontinalis species. Sci Total Environ 345:13–21
Brown MB, Forsythe AB (1974) Robust tests for the equality of variances. J Am Stat Assoc 69:364–367
Cesa M, Nimis PL, Buora C, Lorenzonetto A, Pozzobon A, Raris M, Rosa M, Salvadori M (2014) Moss bags as sentinels for human safety in mercury-polluted groundwaters. Environ Sci Pollut Res 21:6714–6722
Davies TD (2007) Sulphate toxicity to the aquatic moss, Fontinalis antipyretica. Chemosphere 66:444–451
Dazy M, Masfaraud JF, Férard JF (2009) Induction of oxidative stress biomarkers associated with heavy metal stress in Fontinalis antipyretica Hedw. Chemosphere 75:297–302
de Traubenberg CR, Ah-Peng C (2004) A procedure to purify and culture a clonal strain of the aquatic moss Fontinalis antipyretica for use as a bioindicator of heavy metals. Arch Environ Con Tox 46:289–295
Díaz S, Villares R, Carballeira A (2012) Uptake kinetics of As, Hg, Sb, and Se in the aquatic moss Fontinalis antipyretica Hedw. Water Air Soil Poll 223:3409–3423
Díaz SR, Villares R, Vázquez MD, Carballeira A (2013) Physiological effects of exposure to arsenic, mercury, antimony and selenium in the aquatic moss Fontinalis antipyretica Hedw. Water Air Soil Poll 224:1659–1673
Fernández JA, Vázquez MD, López J, Carballeira A (2006) Modelling the extra and intracellular uptake and discharge of heavy metals in Fontinalis antipyretica transplanted along a heavy metal and pH contamination gradient. Environ Pollut 139:21–31
Jasion M, Samecka-Cymerman A, Kolon K, Kempers AJ (2013) Tanacetum vulgare as a bioindicator of trace-metal contamination: a study of a naturally colonized open-pit lignite mine. Arch Environ Con Tox 65:442–448
Kabata-Pendias A (2001) Trace elements in soils and plants. CRC Press, Boca Raton
Kłos A, Rajfur M, Wacławek M, Wacławek W, Wünschmann S, Markert B (2010) Quantitative relations between different concentrations of micro- and macroelements in mosses and lichens: the region of Opole (Poland) as an environmental interface in between Eastern and Western Europe. Int J Environ Health 4:98–119
Krems P, Rajfur M, Wacławek M, Kłos A (2013) The use of water plants in biomonitoring and phytoremediation of waters polluted with heavy metals. Ecol Chem Eng S 20:353–370
Ladislas S, El-Mufleh A, Gérente C, Chazarenc F, Andrès Y, Béchet B (2012) Potential of aquatic macrophytes as bioindicators of heavy metal pollution in urban stormwater runoff. Water Air Soil Poll 223:877–888
Legendre P, Legendre L (1998) Numerical ecology, second English ed. Developments in environmental modelling, Elsevier, Amsterdam
Lithner G, Holm K, Borg H (1995) Bioconcentration factors for metals in humic waters at different pH in the Ronnskar area (N. Sweden). Water Air Soil Poll 85:785–790
López J, Vazquez MD, Carballeira A (1994) Stress responses and metal exchange kinetics following transplant of the aquatic moss Fontinalis antipyretica. Freshwater Biol 32:185–198
Łuszczkiewicz A (1987) Odzysk minerałów ciężkich z piasków szklarskich kopalni „Osiecznica” (Recovery of heavy minerals from glass sand tailings from Osiecznica mine). Fizykochemiczne Problemy Mineralurgii 19:309–319 [Engl summ]
Lytle CM, McKinnon CZ, Smith BN (1994) Manganese accumulation in roadside, soil and plants. Naturwissenschaften 81:509–510
Machajski J (2012) Modelling spillway flow conditions at Złotniki storage reservoir on the Kwisa river. Studia Geotech Mech 34:35–49
Maiti SK (2007) Bioreclamation of coalmine overburden dumps—with special emphasis on micronutrients and heavy metals accumulation in tree species. Environ Monit Assess 125:111–122
Markert BA, Breure AM, Zechmeister HG (2003) Definitions, strategies and principles for bioindication/biomonitoring of the environment. In: Markert BA, Breure AM, Zechmeister HG (eds) Bioindicators & biomonitors principles, concepts and applications. Elsevier Science Ltd, Oxford
Marks L, Ber A, Gogołek W, Piotrowska K (2006) Geological map of Poland. Państwowy Instytut Geologiczny, Warszawa
Martins RJE, Boaventura RAR (2002) Uptake and release of zinc by aquatic bryophytes (Fontinalis antipyretica L. ex. Hedw.). Water Res 36:5005–5012
Martins RJE, Pardo R, Boaventura RAR (2004) Cadmium(II) and zinc(II) adsorption by the aquatic moss Fontinalis antipyretica: effect of temperature, pH and water hardness. Water Res 38:693–699
Mouvet C, Cordebar P, Gallisot B (1986) Evaluation de rejets de micropoluants minéraux (métaux lourds) et organiques (organochlorés) par dosages dans les mousses aquatiques’. XIX Journées de l’Hydraulique, Paris
Muszer A, Łuszczkiewicz A (2006) Mineralogical characteristics of accessory minerals from Osiecznica deposit, SW Poland. Physicochem Probl Mi 40:77–88
Najbar B, Szuszkiewicz E, Zieleniewski W (1999) Wody środkowego nadodrza [Waters of the mid Odra]. B-Art-Ek, Zielona Góra [in polish]
Nazzal Y, Rosen MA, Al-Rawabdeh AM (2013) Assessment of metal pollution in urban road dusts from selected highways of the Greater Toronto Area in Canada. Environ Monit Assess 185:1847–1858
Palmer SCJ, van Hinsberg VJ, McKenzie JM, Yee S (2011) Characterization of acid river dilution and associated trace element behavior through hydrogeochemical modeling: a case study of the Banyu Pahit River in East Java, Indonesia. App Geochem 26:1802–1810
Pekka L, Halmeenpää H, Ecke F, Vuori KM, Mokrotovarova O, Öhlander B, Ingri J (2008) Assessing pollution in the Kola River, northwestern Russia, using metal concentrations in water and bryophytes. Boreal Environ Res 13:15–30
Rasmussen G, Andersen S (1999) Episodic release of arsenic, copper and chromium from a wood preservation site monitored by transplanted aquatic moss. Water Air Soil Pollut 109:41–52
Rau S, Miersch J, Neumann D, Weber E, Krauss GJ (2007) Biochemical responses of the aquatic moss Fontinalis antipyretica to Cd, Cu, Pb and Zn determined by chlorophyll fluorescence and protein levels. Environ Exp Bot 59:299–306
Remon E, Bouchardon L, Le Guédard M, Bessoule JJ, Conord C, Faure O (2013) Are plants useful as accumulation indicators of metal bioavailability? Environ Pollut 175:1–7
Samecka-Cymerman A, Kolon K, Kempers AJ (2005) A comparison of native and transplanted Fontinalis antipyretica Hedw. as biomonitors of water polluted with heavy metals. Sci Total Environ 341:97–107
Sarris A, Kokinou E, Aidona E, Kallithrakas-Kontos N, Koulouridakis P, Kakoulaki G, Droulia K, Damianovits O (2009) Environmental study for pollution in the area of Megalopolis power plant (Peloponnesos, Greece). Environ Geol 58:1769–1783
Say PJ, Whitton BA (1983) Accumulation of heavy metals by aquatic mosses. 1. Fontinalis antipyretica Hedw. Hydrobiologia 100:245–260
Siebert A, Bruns I, Krauss GJ, Miersch J, Markert B (1996) The use of the aquatic moss Fontinalis antipyretica L. ex Hedw. as a bioindicator for heavy metals. Sci Total Environ 177:137–144
StatSoft Inc (2011) STATISTICA (data analysis software system), version 10. www.statsoft.com
Suchara I, Sucharova J, Hola M, Reimann C, Boyd R, Filzmoser P, Englmaier P (2011) The performance of moss, grass, and 1- and 2-year old spruce needles as bioindicators of contamination: a comparative study at the scale of the Czech Republic. Sci Total Environ 409:2281–2297
Valavanidis A, Fiotakis K, Vlahogianni T, Bakeas EB, Triantafillaki S, Paraskevopoulou V, Dassenakis M (2006) Characterization of atmospheric particulates, particle-bound transition metals and polycyclic aromatic hydrocarbons of urban air in the centre of Athens (Greece). Chemosphere 65:760–768
Vaughan IP, Ormerod SJ (2005) Increasing the value of principal components analysis for simplifying ecological data: a case study with rivers and river birds. J Appl Ecol 42:487–497
Vázquez MD, Wappelhorst O, Markert B (2004) Determination of 28 elements in aquatic moss Fontinalis antipyretica Hedw. And water from the upper reaches of the river Nysa (Cz, D), by ICP-MS, ICP-OES and AAS. Water Air Soil Poll 152:153–172
Vázquez MD, Villares R, Carballeira A (2013) Biomonitoring urban fluvial contamination on the basis of physiological stress induced in transplants of the aquatic moss Fontinalis antipyretica Hedw. Hydrobiologia 707:97–108
Vuori KM, Helisten H (2010) The use of aquatic mosses in assessment of metal pollution: appraisal of type-specific background concentrations and inter-specific differences in metal accumulation. Hydrobiologia 656:99–106
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107
Yoshimura E, Sato N, Nishizawa NK, Satake K, Mori S (2000) Accumulation of metals in the cell walls of the liverwort Scapania undulata. J Environ Sci Heal A35:837–847
Zar H (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River
Acknowledgments
We thank Leszek Rudecki for his help in the chemical analyses. This research was supported by University of Wrocław, grant no. 2024/M/KEBOŚ/12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Céline Guéguen
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kosior, G., Samecka-Cymerman, A., Kolon, K. et al. Trace elements in the Fontinalis antipyretica from rivers receiving sewage of lignite and glass sand mining industry. Environ Sci Pollut Res 22, 9829–9838 (2015). https://doi.org/10.1007/s11356-015-4162-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4162-y



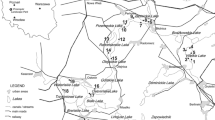

 ; border:
; border:  ; sewage output:
; sewage output:  ; glass sand mine:
; glass sand mine:  , lignite mine:
, lignite mine: 


