Abstract
This study aimed to evaluate the effect of repeated sprint in hypoxia (RSH) training in mask vs. tent system on the physiological parameters associated with the cyclist’s performance. Sixteen well-trained cyclists (VO2max 66 ± 5.9 mL/kg/min) participated in a randomised and two parallel groups design. Participants were assigned to different hypoxia methods [RSHMask (n = 8) vs RSHTent (n = 8)]. The sprint number and power output were measured during a repeated sprint test to failure before and after the effect of eight sessions of RSH. In addition, the following physiological parameters were evaluated: oxygen consumption (VO2), heart rate (HR), arterial oxygen saturation (SpO2), muscle oxygen saturation (SmO2), lactate and core temperature (CoreT°). Linear mixed models were used for repeated measures (p value < 0.05), and the effect size (ES) between groups was reported. An inter-individual analysis of participants was also reported. There was an increase in sprint numbers in both groups (ES = 0.167, p = 0.023) and an increase in power output (∑w) in the RSHMask group (ES = 0.095, p = 0.038). The RSHMask group showed improvement in VO2 recovery (ES = 0.096, p = 0.031) and SmO2 desaturation % (ES = 0.112, p = 0.042) compared to the RSHTent group. Likewise, 50% of the participants in RSHTent showed adaptations to withstand higher T°Core (+ 0.45°), and eight participants showed lactate decreases between 2.9 and 3.1 mmol/L (−24%) after RSH in both groups. Generally, RSH improves the cyclist’s performance, whether the mask or tent method is used. However, RSHTent has the advantage of causing adaptations in T°Core, whilst RSHMask improves anaerobic performance in the oxygenation of peripheral muscles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to low oxygen levels during training sessions, or hypoxia training, is commonly used as a performance-enhancing strategy [1]. Hypoxia training can be performed by ascending to high altitudes or with exposure to simulated low oxygen levels at normal altitude levels; this is known as normobaric hypoxia [2]. Within the implementation of normobaric hypoxia is the Live Low Train High (LLTH) method, which has been studied for its effects on various types of training, including continuous training, interval training and repeated sprint training in hypoxia (RSH), which typically involves short bursts of intense exercise (≤ 30 s) with incomplete rest periods in between [1]. The RSH study has shown increasing interest due to its ability to improve intermittent exercise performance compared to other types of hypoxic training [3, 1]. In addition, RSH seems to be more effective than repeated sprint training in normoxia (RSN) [4].
Some of the adaptations caused by RSH can be explained by the increase in blood flow, which improves the oxygen delivery to fast-twitch muscle fibres. This reduction in substrate-level phosphorylation and intracellular perturbation (e.g. accumulation of Pi and decrease in pH) results in a higher activation rate of fast-twitch muscle fibres compared to slow-twitch fibres, which are more efficient in using oxygen [5]. RSH leads to an increase in Hypoxia-Inducible Factor HIF-1α, which is essential for the gene expression of skeletal muscle cells involved in glycolytic metabolism, mitochondrial turnover and oxygen transport [6]. Similarly, RSH enhances compensatory vasodilation caused by nitric oxide in response to the rise in sympathetic vasoconstriction caused by hypoxia. This process is crucial in maintaining the oxygen supply to the muscles and ensuring that it meets the energy demands [7]. The RSH method also improves vascular endothelial cells, enhancing the angiogenesis and responsiveness of vascular beds [8, 9]. In addition, haematological changes occur, such as an increase in haematocrit that can persist over time in trained athletes (for example, after 2 weeks of detraining) [10]. Likewise, RSH produces a delay in the time to reach the second ventilatory threshold and the blood lactate onset at 4 mmol/L, where the athlete’s fatigue occurs due to metabolic acidosis [11].
These musculoskeletal function adaptations lead to key improvements in cycling performance indicators such as endurance, fatigue resistance and repeated-sprint ability (RSA) [12]. Therefore, RSH is commonly used in endurance sports such as running and cycling, aiming to improve the ability to “sprint after having sprinted,” showing a greater reduction in a “final” sprint of 30 s after a 1 h cycling test [13]. In fact, Etxebarria et al., highlight the need to improve repeated-sprint ability to optimise a final sprint and, therefore, maintain a high level of performance in variable power cycling, commonly found during triathlon races [13]. Overall, if fatigue is delayed during repeated sprint bouts by peripheral adaptations linked to oxygen delivery, one may speculate that the reactivity of the vascular bed is improved. In other words, a delayed fatigue may also be interpreted as a succession of improved recovery phases [14].
Although there is enough evidence supporting the effectiveness of RSH in endurance sports [1], some recent questions have been raised regarding the potential differences in the physiological and performance-related outcomes depending on the hypoxia method used (e.g. tents, masks). The isolation of the tent is linked to the increase in ventilation and condensation of exhaled air, which causes an increase in temperature (< 25 °C), humidity (< 90%) and carbon dioxide (CO2) (< 5000 ppm) [15, 16]. Above these levels, the recommended CO2 level during exercise is < 3000 ppm, the re-inhalation of exhaled air could cause hypercapnic hypoxia and, in combination with normobaric hypoxia, can stimulate ventilation and blood acidification. Likewise, the environmental conditions provoked in the tent during hypoxia training could cause dehydration compared to the mask method. Conversely, a combination of high ambient temperatures and humidity during training could be beneficial for athletes who train and compete in such stressful conditions [17].
To date, only one study has analysed the acute effect of RSH under mask vs tent conditions, and it found no differences in perception or performance variables in cyclists [15, 16]. However, trained athletes can achieve heat acclimatisation by performing five sessions of high-intensity cycling exercises in the high temperatures, which allows them to increase their endurance in hot environments [18]. Moreover, increasing the number of sessions to eight distributed over 4 weeks with 72 h of recovery appears to be the most effective strategy for eliciting molecular adaptations through RSH when utilised as a complement to the cyclist’s regular training regimen [8]. Therefore, it would be relevant to schedule RSH training with a 72 h recovery period to ensure practical applicability for cyclists.
Nonetheless, there exists a dearth of evidence regarding the physiological effects on performance when using a tent vs. a mask to induce normobaric hypoxia. Consequently, there is a need to conduct long-term studies between these two methods. The findings of this investigation could furnish insights into the optimal method for RSH and normobaric hypoxia training, thereby aiding coaches and athletes in planning hypoxia training regimes. We hypothesise that prolonged RSH training within tents may compromise physiological adaptations compared to the mask for simulating hypoxia training. Thus, this study aims to evaluate the long-term effect of RSH training on the physiological parameters associated with the cyclist’s performance depending on the hypoxic method used (mask vs. tent).
Methods
Experimental approach to the problem
The RSH has been proposed as a novel method to improve performance; however, the studies have only evaluated the comparison between hypoxia vs.and normoxia conditions. The objective is to compare two normobaric hypoxia systems (mask vs. tents), and examine their respective effects on physiological parameters relevant to cyclists. It was hypothesised that the physiological adaptations are different because the temperature, humidity and CO2 increase inside the tent. The dependent variables in this study are changes in performance parameters: the number of supramaximal sprints, total power sum, and physiological parameters: VO2, VO2max, HR, SmO2, SpO2, lactate and T°Core and the independent variables are the conditions of hypoxia systems (mask and tent). To achieve this goal, a randomised and parallel-groups study design with a convenience sample was used (see Fig. 1.) [19]. Participants were allocated a unique trial identifier number based on the recruitment sequence. Due to the relatively small sample size, permuted-block randomisation was used via web-generated random numbers to ensure an equal sample size [20]. This study had two phases: experimental and control. In a fist phase, participants (n = 16) performed only one of the experimental conditions, RSHMask or RSHTent. Experimental phase consisted of two sessions per week for 4 weeks of each of the two conditions, a total of eight participants were assigned to each experimental group. After a washout period of 2 months [21] to allow RSH detraining, in a second phase, all participants (n = 16) joined a control condition group during another 4 weeks. Likewise, this intervention was conducted at a time during the general preparation season, to avoid the adverse effects of detraining in hypoxia. Study protocol was based on previously published methods [22] and modified accordingly.
Subjects
A total of 16 well-trained cyclists [23] were recruited (age 25 ± 5.8 years, weight 63 ± 9.2 kg, height 175.1 ± 8.6 cm, maximal oxygen consumption (VO2max) 69 ± 5.9 mL/kg/min, training frequency 6.5 ± 0.5 times a week). The sample was convenience-sampled, subsequent power calculation was performed to determine the effect size with eight participants. The same participants were enrolled in one experimental group and the control group (crossover design). Also, they competed in official races organised by the Spanish cycling federation at the national level. As inclusion criteria, participants should train for at least 15 h/week and be accustomed to high-intensity training. The participants were required to have no prior experience with acute hypoxic training (ascending over 1500 m) or mountain sickness in the past 3 months. Two individuals were excluded from the study due to reported neuromuscular injuries in the last 6 months. All participants were informed about the study’s objectives, the protocol details, their rights during participation, and any potential risks associated with the experimental protocol. The study followed biomedical guidelines based on the Declaration of Helsinki. In addition, the protocol was reviewed and approved by the Institutional Review Board of the University (Reg. Code 174/2020). Participants were asked to avoid intense, prolonged, or strenuous exercise; alcohol consumption; and caffeine for at least 24 h before all tests (pre and post experimental period and pre- and post-control period). During all the study, participants continue their regular cycling training established by the coaches (i.e. usual training load).
Procedures
An electronic scale (VitalControl, Hans Dinslage GmbH, Uttenweiler, Germany) was used to assess total body mass (kg) with a precision of 0.1 kg. Stature was measured using a wall stadiometer. Anthropometric variables were measured following the International Society for the Advancement of Kinanthropometry guidelines. The VO2max was obtained using a gas analyser (Metalyzer 3b, CORTEX Biophysik GmbH, Leipzig, Germany) via an incremental test of 5 min warm-up at 50 W followed by 1 min rest. Then participants cycled at 60 W, and the work rate was increased by 30 W every 3 min until exhaustion. Achievement of VO2max was verified based on the incidence of the plateau phase (two successive maxima within 150 mL/min−1, averaging the data every 5 s) reached in oxygen consumption (VO2). These measurements were carried out 1 week before starting the intervention in a single session. All testing protocols were performed at sea level in a temperature-controlled laboratory (20–3 °C).
Experimental conditions
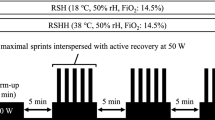
Each session was completed in a normobaric environment using a mask (RSNMask and RSHMask) and a tent (RSNTent and RSHTent). An altitude system (CAT-12, Louisville, Colorado) was used to simulate hypoxia at a fraction of inspired oxygen (FiO2) calibrated at FiO2 of 14.3%, corresponding to 3700 m and at moderate to high altitudes. Hypoxia was produced by a generator using a semi-permeable filtration membrane (nitrogen filter technique) connected to a waterproof face mask or waterproof tent, depending on the hypoxia method. The mask method included a neoprene harness that guaranteed ideal support and attachment for exercise in hypoxia (See Fig. 2). The tent method (CAT-430, Louisville, CO, USA) used for the RSHTent conditions had a size of 9 m2, and a 15 m2 room was used for the RSHMask conditions (See Fig. 2). FiO2 was constantly monitored throughout the sessions using a portable device (HANDI + , Maxtec, Salt Lake City, UT, USA). Likewise, the control of SpO2 was used during the two conditions: mean value of all sessions of RSHMask (SpO2 = 88 ± 4%) and for RSHTent (SpO2 = 86 ± 5%) (p value = 0.362). The external environmental conditions were controlled, so the training sessions were carried out in similar situations (21–24 °C and 45–55% relative humidity). After a 4 week washout period, as a reference to regular training conditions, the control group maintain regular training sessions and habitual load [22].
Repeated sprint training in hypoxia intervention
Participants executed a warm-up of 9 min at moderate intensity at 100 watts and 80–90 rpm in each training session, followed by a min post block with a 10 s submaximal sprint and a 50 s active recovery. The RSH consisted of three bouts of five series of 10 s repetitions (all-out sprints), with an active recovery of 20 s between repetitions and 180 s between series. Final recovery of 7 min was given for a total of 30 min per session. At least 72-h recovery was established between sessions, and all tests were performed at a similar time to avoid circadian cycle effects.
The protocol was performed in an isolated laboratory under controlled settings. Indoor environmental conditions were controlled with a digital logger (Green Eye, TechGrow, The Hague, The Netherlands) with an internal stable non-dispersive infrared sensor for CO2 detection, indoor temperature (°C) and relative humidity (%) (SenseAirTM, Delsbo, Sweden). The equipment was calibrated through an automatic reference calibration function and measured the amount of infrared light of a specific wavelength absorbed by air, which was then obtained to calculate the CO2 concentration. The recorder was placed 1 m away, next to the cyclist, at the height of 1 m. The mean values of the RSHMask group were CO2: 789 ± 400 ppm, temperature: 21 ± 3 °C, relative humidity: 34 ± 10% vs RSHTent group, were CO2: 7476 ± 2200, temperature: 26 ± 5, and relative humidity: 94 ± 19 (p value = 0.001) during the RSH sessions (See Fig. 2). Finally, 72 h before the intervention, the participants carried out a familiarisation session in a normoxic environment without any hypoxia method.
Pre–post assessments: repeated-sprint ability (RSA)
This test was used to evaluate the RSH effects on performance, before and after each condition and before and after control period. The variables were measured during a RSA test to failure. Power output (W), relative power output (W/kg) and cadence (rpm) were measured, coupling the cassette of each participant’s bicycle to a cycle ergometer with electronic resistance (CycleOps® Hammer, Madison, WI, USA). The smart trainer assessed cadence metre and power metre were paired to a smartwatch for future analysis (Forerunner 735xt, Garmin™, Olathe, KS, USA) and then analysed using software (Garmin Connect, Garmin, Olathe, KS, USA). Before starting the test, the participants completed the same warm-up as during the training sessions. They then performed two 10 s single sprints to obtain maximal baseline power that was calculated from the best power of the two sprints, followed by a 3-min active recovery. The RSA test consisted of repeating maximal sprints of 10 s and rests of 20 s (ratio 1:2). The first two sprints were found to be at least > 95% of the best warm-up sprints. Task failure was declared when peak power fell below 70% of peak power output, or a pedalling cadence of at least 70 rpm could not be achieved in the first half of the sprint (Raberin et al., Millet 2022). Strong verbal encouragement was provided, and there was no indication of the number of sprints performed.
The total number of sprints and mean power output (average of all sprints) were recorded. The following equation determined the fatigue index (FI) [24, 12]:
Cardiopulmonary variables: Gas exchange was recorded continuously throughout the repeated sprint test through a breath-to-breath system and ventilatory gas exchange analysis (Metalyzer B3, Cortex, Leipzig, Germany). Standardised calibration procedures were performed before the exercises started, as the manufacturer recommended. They included turbine calibration with a standard 3000 mL syringe for airflow (Metamax 3B, Cortex Biophysik), ambient air calibration with an assumed concentration of 20.94% O2 and 0.03% CO2 gas calibration with a certified commercial gas preparation (16% oxygen, 5% carbon dioxide) and delayed calibration to ensure accurate readings during testing and verify alignment between gas flow and gas concentrations. During RSA, all cardiorespiratory measurements were automatically filtered for aberrant data points, interpolated at 1 s intervals, and time synchronised. The following variables were calculated: the maximum values of VO2 during the 10 s sprint, minimum values of VO2 during the 20 s of sprint recovery (VO2 recovery) and the VO2 max were recorded as the value of VO2 plus high obtained throughout the test.
The arterial oxygen saturation (SpO2): The SpO2 was recorded using a finger oximeter (Checkme O2, Viatom Technology Co., Shenzhen, China). Several studies have established the reproducibility and validity (intraclass correlation coefficient = 0.88) for this measurement method for saturations above 80% compared to arterial blood gas measurements [25]. The average for each sprint set was considered for analysis.
Local muscle oxygen saturation (SmO2): The SmO2 was assessed with a near-infrared spectroscopy (NIRS) device (MOXY, Fortiori Design LLC, Minneapolis, Minnesota, USA), which is valid for measuring SmO2 (ICC: r = 0.773–0.992) [26]. It was attached firmly to the belly of the right vastus lateralis muscle (midway between the lateral epicondyle and greater trochanter of the femur) using a dark elastic strap to avoid light contamination and movement artefacts. The vastus lateralis was selected based on previous evidence and considering the role of this muscle in cycling [27]. The skinfold thickness at the NIRS measurement site (VL) was measured using a skinfold calliper (Harpenden Ltd.) to ensure that the skinfold thickness was < 1/2 of the distance between the emitter and the detector (25 mm). The desaturation minimum peak (SmO2 decrease) and maximum peak oxygenation (SmO2 recovery) were measured for each sprint, and the mean of the entire sprint trial was considered for analysis. This technology allows the SmO2 evaluation, taking into consideration the relative change of total haemoglobin (tHb) and the interaction between O2Hb and HHb with the following equation:
This was calculated by quantifying the variation in optical transmission by sequentially emitted light waves (630–850 nm) from light-emitting diodes into the tissue and recording the amount of light received. Using an algorithm, the system determined the amount of light absorbed at wavelengths corresponding to oxygenated and deoxygenated Hb using the Beer–Lambert law and tissue light propagation model processes. The raw muscle O2 saturation signal was treated with a soft spline filter to reduce the noise created by movement [28] using a Minitab 19 (Minitab, Inc, State College, PA; www.minitab.com, USA).
Heart rate (HR): The HR (bpm) for each sprint set was recorded using a heart rate monitor chest band (HRM-Tri, Garmin™, Olathe, KS, USA). The heart rate strap was placed at the level of the xiphoid and was adjusted using a strap system. HR data obtained were HR peak and recovery for each repeated sprint.
Temperature CORE (T°Core): T°Core was measured with a specific non-invasive sensor used to assess core body temperature (CORE®, Green TEG, Switzerland) [29]. The CORE sensor is commercially available and includes a new thermal energy transfer sensor (a heat flow sensor) that determines core temperature using machine learning algorithms based on heat flow detection and skin temperature, as well as when exercising. According to the manufacturer’s instructions, this sensor should be worn on the torso/chest approximately 20 cm below the armpit using a heart rate monitor strap. Data stored on the device can be downloaded to the CORE Android or iOS app for further analysis. The manufacturer describes the accuracy of the CORE device as ± 0.26 °C. The limits of agreement during training are − 0.30 °C and 0.42 °C, and during high-intensity exercise, it can vary from 0.22 ± 0.33 to 0.33 ± 0.33 °C compared to rectal temperature. The variables HR, SmO2, T°Core along with power were linked to a smartwatch (Forerunner 735xt, Garmin™, Olathe, KS, USA) and later analysed in the Garmin data cloud.
Lactate concentration (mmol/L) was determined with a portable lactate test metre (Lactate Pro 2, Arkray Factory, Inc., Amstelveen, The Netherlands) 3 min before and 3 min after the repeated sprint test. The difference was obtained as the total value of lactate. The equipment was calibrated following the manufacturers’ guidelines. The mean data of the entire test were used for data analysis.
Statistical analysis
All data were analysed and systematised using the Statistical Package for the Social Sciences (SPSS v.22.0, IBM, Chicago, IL, USA). Results were reported using the mean and standard deviation. The normality of the data was confirmed by the Shapiro–Wilk test. A sample power analysis (G*Power: version 3.1., University of Dusseldorf, Germany) was performed to determine the effect size to detect significant changes in a group of eight participants per condition, based on using an [alpha] of 5%, a 1-[beta] of 80%, and an effect size of 0.743 based on peak power (w/kg) values from the study performed previously [15, 16]. Differences in performance and physiological parameters were analysed using linear mixed models for repeated measures. As a dependent variable, the variable of interest (watts, VO2, HR, SmO2, T°Core, etc.) was entered into the model. They were entered as fixed effects to investigate the impact of hypoxia methods, the evaluation time (pre and post), and the interaction between them. When the model detected a significant difference of p value < 0.05, the p values were interpreted with care as descriptive weights of evidence rather than as confirmatory claims, and a Bonferroni post hoc was applied to detect the differences between the groups. The magnitude of change (Δ%) for each variable was reported with 95% limits of agreement and effect size (ES) via omega squared (ωp2) was used to qualify and quantify the differences in the magnitude of the factors (interaction, within-subjects factor (time main) and between-subjects factor (group main)). ES interpretation of results was as follows: < 0.01, trivial; > 0.01, small; > 0.06, moderate; and > 0.14, large [30]. Also, the minimum detectable change (MDC) was within 95% limits of agreement. The variables selected in the MDC analysis were based on the criteria of ES, significance p value < 0.05 and interest of the researchers in the variables of interest by the researchers were evaluated. The MDC was calculated using the following formulae [31]:
To further assess the substantial changes that can be considered “real”, the MDC was used. A calculated signal:noise (S:N) rate was then determined [32]. For a variable to be considered capable of detecting a “real” change, the post-intervention group mean change (signal) must be greater than the MDC (noise), as indicated by a ratio > 1. The S:N rate indicated the variable strength by comparing the different hypoxic conditions and was plotted for inter-individual analysis to obtain the number and percentage of participants who responded to the RSH effect.
Results
The 16 participants were divided into 2 groups of 8 participants. An external investigator carried out the statistical analysis to avoid identifying the group to which they were assigned. All participants complied with the intervention, completing a mean of 63 RSHMask sessions (95% CI 62–64) and 60 RSHTent sessions (95% CI 58–63), with a p value = 0.984, indicating no significant difference in compliance between groups. Therefore, compliance was generally good, as reflected in the exercise diary. Blinding was successful because no subject dropped out of the hypoxia training programme. The only recorded adverse effect was that two participants in the first RSHMask session did not complete the full session due to complaints of mask dizziness, and therefore was not considered for analysis. A G-power of 0.932 was detected for this study based on the maximum power (W/kg) values.
Table 1 displays the difference in environmental conditions between RSHMask and RSHTent throughout the 4-week intervention. Higher levels of CO2 (Δ = 78%) and relative humidity (Δ = 64%) are observed in RSHTent than in RSHMask, indicating adverse environmental conditions in the latter. The environmental temperature did not differ between conditions.
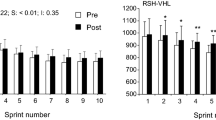
Table 2 shows the changes before and after the intervention of the variables of power, relative power, fatigue index and power sum. An increase in the number of sprints was observed after the intervention in both RSH hypoxia groups, with a magnitude change of 25% (p value = 0.001) in RSHMask and a magnitude change 27% (p value = 0.001) in RSHTent. There was also an increase in the power sum in the RSHMask group, with a magnitude of 2% (p-value = 0.002). There were no pre–post differences in the other variables. Likewise, the effect between groups was not significant in any variable related to power and accumulated fatigue.
Table 3 shows the changes before and after the intervention in the physiological variables of oxygen consumption (VO2 max, VO2, SpO2 and SmO2). Higher values of VO2 recovery were observed in RSHMask compared to RSHTent (magnitude change of 24% vs. − 1.5% p value: 0.042) and RSHMask compared to the control group (magnitude change of 24% vs. 4.3% p value: 0.013), VO2 recovery obtained a moderate effect size in the main group (ES = 0.096).
A greater muscle desaturation is also observed in the RSHMask and RSHTent groups compared to CG (magnitude change of 10% vs. 0.4, p value: 0.007 and p value: 0.011) with a moderate effect size in main group (ES = 0.112). Also, higher values in muscle resaturation were observed in RSHMask compared to RSHTent (pre: 37 ± 12 vs. 23 ± 13 p value: 0.004, and post: 41 ± 18 vs. 32 ± 14 p value: 0.032). However, the change magnitude was greater in the RSHTent compared to the RSHMask (28% vs. 10%, p value: 0.003). There was a moderate effect size in main group (0.079) and time main (0.073). Still, muscle oxygenation differences were not significant (p value > 0.05). Concerning the VO2, VO2 max, and SpO2, there were no differences in time or the condition interaction for the hypoxic methods.
Table 4 presents the changes before and after the intervention in heart rate, lactate and T°Core. No statistically significant changes were observed. However, a decreasing trend in lactate was shown in the RSHMask (change magnitude of −12% p value: 0.276) and RSHTent (change magnitude of −18% decrease = 15% p value: 0.193), with no difference in change magnitude between groups. A small effect size (ES = 0.058) was obtained. There is also a trend of an increase in T°Core values in the RSHMask group with an increase of 1.1% (p value: 0.417) and in the RSHTent group with an increase of 0.5% (p value = 0.146) and moderate effect sizes time main (ES = 0.081), but without a statistically significant difference (p value < 0.05) in change magnitude. Likewise, no significant change was observed in the HR variables.
Table 5 shows the inter-individual response of the physiological parameters. An increasing trend was observed in sprint numbers, where three participants (38% sample) responded to an increase of MDC = 3 (+ 29%) sprints of the RSHMask. Likewise, five participants (62% sample) responded to an increase of MDC = 3 (+ 31%) sprints of the RSHTent. A greater response of sprint numbers in RSHMask (S:N = 3.87) was observed compared to RSHTent (S:N = 3.78). The inter-individual response of the power sum (w) shows that the same three participants responded to an increase of MDC = 2625 w (+ 39%) in the RSHMask group, but no change in performance was observed. A real change was observed in the RSHTent group with an MDC = 3399 w (+ 59%). A greater response of the power sum in RSHMask (S:N = 2.99) was observed compared to the effect of RSHTent (S:N = 1.92).
Regarding the inter-individual response of VO2max, two participants responded to an increase MCD = 4.4 (ml/kg/min) (+ 25%) in RSHMask compared to one participant in RSHTent with MDC = 4.2 (ml/kg/min) (+ 12%), with a lower S:N in RSHMask than in RSHTent (8.09 vs. 8.72, respectively). In the inter-individual response of VO2 recovery, three participants responded to an increase MDC = 0.76 (L/min) (+ 19%) in RSHMask compared to two participants with an increase MDC = 0.70 (L/min) (+ 22%) in RSHTent, with a higher S:N in RSHMask than in RSHTent (5.46 vs. 4.59, respectively). In the inter-individual response of muscle desaturation (%), two participants responded to the decrease MDC = 9 SmO2 (−52%) in the RSHMask group. No real change response was observed in the RSHTent group with MDC = 5 SmO2 (−53%). Likewise, RSHMask showed a higher S:N than RSHTent (2.04 vs. 1.98, respectively). Similarly, in the muscle oxygenation response (%), the same two participants in RSHMask showed an increase in MDC = 13 SmO2 (+ 33%). No real change response was observed in RSHTent, with MDC = 12 SmO2 (+ 43%). Likewise, RSHMask showed a higher S:N than RSHTent (3.12 vs. 2.70, respectively).
In the inter-individual response of the T°Core, changes were observed only in the RSHTent group with four participants (50% sample), and no participant in RSHMask responded to any real change. The S:N was higher in RSHTent than in RSHMask (84.27 vs 51.96, respectively). In the inter-individual response of lactate (mmol/L), five participants in RSHMask showed a decrease with a MDC of 3.1 mmol/L (−24%), whilst three participants in RSHTent showed a reduction with a MDC of 2.9 mmol/L (−24%). The S:N was higher in RSHMask than in RSHTent (3.84 vs 3.78, respectively). Finally, no participant responded to the effect of any hypoxia method in HR response, and the S;N was higher in RSHMask than in RSHTent (30.72 vs 17.56, respectively).
Discussion
This study discovered differences in the effects of RSH using a mask vs. the tent method on physiological parameters related to cyclist performance. Key findings include an increase in sprint numbers in both groups (RSHMask and RSHTent), but only a slight improvement in power output and peripheral SmO2 values in RSHMask group. In addition, using the RSHTent showed that 50% of the sample obtained adaptations to support higher T°Core during repeated sprint exercise, potentially due to the conditions present in the tent method.
An increase in the sprint numbers was observable in both groups, with RSH having the ability to increase fatigue resistance capacity and lengthen task failure in repeated sprints [4, 8]. However, it is accepted to consider the larger changes that occur in repeated sprint training in equivalent oxygen-deprived environments at altitudes above ~ 3000 m or FIO2 < 14.4% than at lower elevations [33]. Although in our study, only the RSHMask group obtained an increase in mean power (+ 37%, Δ = 2%), power can be maintained with the RSHTent but not increased with RSHMask [34]. Increased power output is related to the ability to recruit more fast contractile (FT) fibres, and FT is known to be essential for power output as intensity increases [35]. Some recent evidence [36] demonstrated that RSH training significantly increased the number of maximal cycling sprints, although mean power output remained unchanged compared to equivalent normoxic training. These authors explain that the possible reason for the different results may be due to methodological differences, including training status, type of sport and degree and frequency of hypoxia, as previously described elsewhere [1].
Some studies have shown the differences between hypoxia and normoxia at different work ratios of 1:2 [12]. Similarly, our study worked at a ratio of 10:20 s, but there was no difference (see Table 1). In the same context, within the indices studied to quantify the fatigue influence during repeated sprint exercise, the FI % can be used. It is possible that the FI% is not sensitive to failure at a 70% intensity decrease because no difference between the groups was found in this study. However, we must not ignore that a small change in individual power produces a large change in time to exhaustion [37], which can influence the FI% values. Following the context of RSA’s, regarding the effect of the RSH session numbers, the exact session numbers that cause adaptations are still up for debate. Still, less than six sessions seem to cause no adaptations [38]. Another study similar to ours found that after six to eight RSH sessions, the number of sprints before task failure increased by 38% in well-trained cyclists [8] during an RSA protocol of ‘open loop’. In our study, + 29% RSHMask and + 37 RSHTent were observed in well-trained cyclists (RSHMask 9 ± 2 to 12 ± 3 and RSHTent 8 ± 3 to 11 ± 4). In addition, the difference in power observed between the hypoxic groups may be attributed to the athletic abilities of the participants, as some individuals can maintain high levels of energy production (e.g. developed extramitochondrial energy systems) and recover quickly (e.g. high maximal ¨aerobic¨ power), thereby delaying reliance on extramitochondrial energy and glycolysis during repeated sprints in O2-deficient environments [39], this was demonstrated in this study with a higher VO2 recovery both pre and post trend maintained (RSHMask 3.74 ± 0.93 to 4.16 ± 0.81 L/min and RSHTent 3.27 ± 0.65 to 3.22 ± 0.95 L/min).
In the same context, it was found that only RSHMask exhibited a significant improvement in VO2 recovery and was different with RSHTent and CG, but RSHTent was not different with CG. This shows that limiting oxygen within a mask system can be more effective for better mitochondrial energy-based performance during repeated sprinting [40]. The VO2 recovery influences the phosphagen system restoration, blood lactate removal and improvement of greater oxygen extraction for the following sprint [41], according to Gregory et al. 2005. As per Fick’s principle, oxygen uptake (VO2) is determined by the product of cardiac output (heart rate × stroke volume) and the arteriovenous oxygen difference. Therefore, the improved VO2 recovery in this study may be due to increased cardiac output and oxygen transport to peripheral muscles, as evidenced by the increase in blood flow [42]. Although most studies focus on the increase in VO2 max or VO2 peak as the central performance parameter for each sprint, no significant changes were observed in our study. However, the parameters of pulmonary oxygenation are not the only triggering factors in a greater power and sprint numbers [43]. At the level of peripheral metabolism, a greater SmO2 recovery can be determined, a phenomenon known as muscle oxygen restoration [15, 16]. The RSHMask and RSHTent groups showed a trend towards lower muscle desaturation compared to the CG. Still, it was only a trend from pre- to post-intervention, indicating no significant difference in the hypoxic effect. The ES has been shown only in the main condition group (ES = 0.112) because they are different with the CG. Therefore, it can be determined that RSH improves muscle desaturation. Concerning muscle desaturation and resaturation, a change was observed in two participants of RSHMask. Together with the VO2 recovery results, this supports the adaptations observed only in the RSHMask group, although it cannot be concluded how the different types of system affect SmO2. It can be intuited that the increase in blood flow and muscle’s vasodilator capacity after RSH are responsible for improvements in muscle desaturation and resaturation [15, 16].
Since the desaturation muscle is similar to phosphocreatine restoration, RSH may lead to beneficial adaptations at the muscle level, along with improved blood perfusion, which may result in further enhancements in RSA. For example [8, 44], it has been shown that the use of muscle O2 is reflected by the greater use of O2 by fast-twitch fibres. Although the power decrease is mainly due to neural failure in short-duration sprints, this power deficit represents a protective mechanism against the progressive muscle desaturation observed in this mediated by afferents III and IV [45]. Likewise, inducible changes in muscle can be visualised with muscle desaturation by SmO2, which is a direct measure of reoxygenation (O2HHb), HIF-1α and VEGF factors [46, 47]. The inter-individual response of cyclists will depend on the haemodynamic of the capillary beds to maintain the oxygen supply and the nutrients transport to the skeletal muscle [48]. Likewise, an improvement in oxygen contribution is measured by nitric oxide within the muscle (nNOS), which is necessary to optimise muscle perfusion [49] A blood flow increase due to the hyperaemic response is associated with the ability to remove and buffer H+ ions and the ability to replace intramuscular PCr [50]. Therefore, this study supports the NIRS use as a performance factor in RSH tests and cyclist performance.
Similarly, SpO2 did not show any differences between groups or at the intervention time, indicating that RSA may not be the best method to distinguish SpO2 changes during hypoxic training. However, it is hypothesised that SpO2, a variable related to systemic metabolism, may impact performance [51] and be a limiting factor. It should be noted that this statement does not pertain to resaturation, whether it is systemic or localised muscle oxygen during short periods of high intensity [15, 16]. Therefore it is better SpO2 values should be taken into account to measure the adaptations within RSH sessions.
Another factor to highlight from this study is that there was a 24% decrease lactate at the end of the RSA test in eight participants (50% of the total sample). The positive regulation of lactate metabolism may reflect sport-specific mitochondrial performance gains [52]. Essentially, lactate is exchanged by tissues throughout the body [53]. In response to our study, it can be confirmed that the cell-to-cell lactate shuttle and astrocyte-neuron lactate shuttle is the main reason for decreased lactate as an adaptation to training [54, 55]. The rate of ATP demand in exercising skeletal muscle increases, lactate accumulates and efflux of muscle fibres via MCT1 and MTC4 increases within the exercising muscle. Lactate is transported between types of heterogeneous fibres, from glycolytic fibres to oxidative fibres, through MCTs [53].
In inactive glycolytic skeletal muscle fibres, lactate is mainly converted to glycogen, with some oxidised in oxidative fibres. Alternatively, lactate can be transported through the bloodstream to less active or moderately active skeletal muscles, oxidised (CO2 + H2O) in oxidative fibres. In the brain, the astrocyte-neuron lactate shuttle is the process by which astrocytes take up glucose from the microcirculation, converting glucose to lactate via glycolysis [54, 55]. This astrocytic lactate diffuses from the astrocyte to an adjacent neuron via MCT. In the neuron, lactate is oxidised in mitochondria to resynthesise ATP and is used for the cataplerotic production of amino acid neurotransmitters (NT, e.g. glutamate, aspartate, and GABA). Interestingly, HIF-1 and lactate exhibit reciprocal activation, whereby lactate can activate HIF-1 and HIF-1 can promote lactate production [56]. HIF-1 also increases the content of MCT4, the isoform associated with lactate efflux from glycolytic skeletal muscle.
Although it could be reasoned that a high glycolytic activity of the RSAs necessitates a high fraction of extramitochondrial ATP production and the short recovery time would not allow for greater lactate output, due to stimulation of muscle phosphofructokinase (PFK) at through hypoxia, this is possible [57]. PFK is known to be the kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis [58]. Consistent with such assumptions, we found 6 weeks of sprint interval training in hypoxia to increase PFK muscle activity by approximately three times that of an equivalent volume of SIT in normoxia. This finding is in line with previous research showing that high-intensity resistance training in hypoxia causes greater upregulation of muscle PFK mRNA expression [59]. This likely effect is due, at least in part, to elevated HIF-1 activity during hypoxic training. HIF-1 is suggested to stimulate PFK enzyme transcription [60]. Muscle tearing state output is markedly enhanced during high-intensity exercise, most prominently in type II active motor units. Finally, other studies have observed that 8 weeks of RSH caused a decrease in lactate along with an improvement in the glycolytic pathway through normobaric hypoxia stimulation (~ 3850 m) [61, 62]. Therefore, a lactate decrease, but an increase in output power is the main adaptations of RSH in our study.
Currently, an interesting variable is the study of the T°Core due to the impact it can have on performance, subject who exercise in ambient temperatures of 30 °C to 35 °C can increase the acute performance of repeated sprints without harmful heat stress [63]. This effect generally occurs if T°Core is kept below 38.5 °C. However, it is not clear how hypoxic exposure can affect these temperature limits; if the body does not go into a hyperthermic state (> 38.5 °C), adding heat up to 40 °C in an RSH could amplify the decrease in SmO2 and muscle oxygen extraction, and therefore increased blood flow and glycolytic energy utilisation [64, 65]. No major changes were observed in our study in any of the tests. Whilst we do not know the T°Core that was reached inside during RSHTent intervention, our data support future research that measures T°Core during repeated sprints [63]. Similarly, the most critical conditioning variable of the RSHTent is relative humidity (RH%), which is linked to increased ventilation and pulmonary reactive respiratory chemicals related to the local redox state altered by exercise and inflammatory mediators (hydroperoxides, nitrites, malondialdehyde, peroxynitrite, leukotrienes, cytokines, etc.) in the exhaled breath condensate.
Airway dehydration has been suggested as one of the main factors involved in inflammation and oxidative stress at the respiratory level due to physical exercise [66]. Under these conditions, and as a consequence of the increased respiratory flow, the organism is inefficient in saturating the inhaled air with water vapour and tempering it, which leads to dehydration of the respiratory epithelium and the release of local inflammatory factors, generating bronchoconstriction [67, 68]. A physiological response of bronchodilation to exercise helps the adequate health state of the participants. High humidity prevents dehydration and airway hypertonicity associated with increased ventilation during exercise [69]. In recreational cyclists, high environmental humidity prevents the respiratory production of hydrogen peroxide and nitrite induced by intense physical exercise, preserving bronchodilation [70]. Finally, regarding HR, there were no visible and important changes; few studies suggest HR as an adaptation or a difference between RSH groups. The metabolic variables related to O2 seem more determinant than HR in knowing the adaptations to repeated sprint training [50].
Limitations and suggestions
Amongst the main limitations of our study, we found a small sample size. For this reason, heterogeneity of the sample and other individual characteristic leads to a lack in clarity in some physiological variables. However, this study shows that RSH improves the fatigue resistance of repeated sprints; the information is useful for coaches, cyclist trainers and endurance sports. Another limitation is the control of the exercise-rest ratios; it is possible that adding other work ratios, such as 1:4, could have an impact on the T°Core due to the increase in time and energy inside the tent. As suggestions, many sports activities are based on the ability to repeat various efforts over time. The ability to perform these activities appears to depend on the ability to rapidly restore phosphocreatine, ensuring that high rates of muscle work can be maintained over the course of multiple high-intensity bouts. Therefore, we support the use of RSH by them in the different hypoxia methods. In addition, the variables of this study must be interpreted with caution by sports scientists; non-invasive variables such as T°Core and SmO2 are of great potential to identify physiological adaptations and therefore use in RSH studies is recommended.
Practical applications
Based on the outcomes of this study, some practical applications could be given for coaches, athletes and sport scientists. It could be incorporated in normobaric hypoxia training programme of trained athletes to improve their RSA, cardiopulmonary variables, SmO2, T°Core and extra mitochondrial metabolism adaptations. Besides it should be considered using a mask or a tent for normobaric RSH training depending on the specific needs of the athlete or the sport. For example, an RSHMask causes a slight improvement in the peripheral adaptations of SmO2 compared to the RSHTent, this could have an advantage in more ¨anaerobic¨ (extramitochondrial energy-based) tests. Likewise, the exercise in RSHTent causes improvements in the adaptations to withstand higher T°Core than when performing RSHMask, this would have an advantage in competitions in hot and humid climates. When programming RSH, potential risks and side effects of normobaric RSH should be addressed such as fatigue, hypercapnia, headaches or impaired cognitive function and training programmes should be adjusted accordingly.
The monitor of SmO2 during normobaric RSH to track the athlete’s progress and adjust the training programme could be used in future studies and requires a more in-depth analysis. Consequently, combining normobaric RSH with other training modalities such strength training could maximise benefits, and could be studied in future research. Investigate the long-term effects of using different hypoxia methods (mask vs tent) on repeated-sprint ability and physiological parameters associated with cycling. The current study only looked at the effects of eight RSH sessions, so it would be interesting to see if the benefits persist over a longer period. Investigate the effects of normobaric hypoxia on other physiological parameters, such as immune function or hormonal responses. This could help to better understand the overall impact of normobaric hypoxia on athletic performance and recovery. Conduct a cost–benefit analysis of using normobaric hypoxia in training for different sports. This could help coaches and athletes make informed decisions about whether to invest in equipment for normobaric hypoxia training. Develop guidelines for the safe and effective use of normobaric hypoxia in training for athletes. This could include recommendations for the duration and intensity of hypoxic exposure, as well as monitoring of physiological parameters to ensure safety.
Conclusion
In summary, the effect of eight RSH sessions can be evaluated as positive for performance due to increased fatigue resistance of the RSA, as well as adaptations in cardiopulmonary variables, peripheral oxygenation and lactate metabolism, regardless of the hypoxia system used. However, the different hypoxia systems, RSHMask and RSHTent, cause different effects on some internal physiological variables related to the environment. For example, RSHMask leads to a slight improvement in the peripheral adaptations of muscle oxygen transport (SmO2) compared to RSHTent. This could provide an advantage in more anaerobic tests. Conversely, exercise in RSHTent causes improvements in the adaptations to withstand higher TºCore than when using RSHMask, which would provide an advantage in competitions in hot and humid climates. Finally, the use of normobaric hypoxia remains attractive for trained athletes, whether through a mask or a tent.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Millet G, Girard O, Beard A, Brocherie F (2019) Repeated sprint training in hypoxia—an innovative method. Dtsch Zeitschrift für Sportmed. https://doi.org/10.5960/dzsm.2019.374
Lundby C, Millet GP, Calbet JA et al (2012) Does “altitude training” increase exercise performance in elite athletes? Br J Sports Med 46:792–795
Girard O, Brocherie F, Goods PSR, Millet GP (2020) An updated panorama of “living low-training high” altitude/hypoxic methods. Front Sports Act Living. https://doi.org/10.3389/FSPOR.2020.00026
Brocherie F, Girard O, Faiss R et al (2017) Effects of repeated-sprint training in hypoxia on sea-level performance: a meta-analysis. Sports Med 47:1651–1660. https://doi.org/10.1007/s40279-017-0685-3
Faiss R, Girard O, Millet GP (2013) Advancing hypoxic training in team sports: from intermittent hypoxic training to repeated sprint training in hypoxia. Br J Sports Med 47:i45–i50. https://doi.org/10.1136/BJSPORTS-2013-092741
Nava RC, McKenna Z, Fennel Z et al (2022) Repeated sprint exercise in hypoxia stimulates HIF-1-dependent gene expression in skeletal muscle. Eur J Appl Physiol 122:1097–1107. https://doi.org/10.1007/S00421-022-04909-3/FIGURES/4
Casey DP, Joyner MJ (2012) Compensatory vasodilatation during hypoxic exercise: mechanisms responsible for matching oxygen supply to demand. J Physiol 590:6321–6326
Faiss R, Léger B, Vesin J-M et al (2013) Significant molecular and systemic adaptations after repeated sprint training in hypoxia. PLoS ONE 8:e56522. https://doi.org/10.1371/JOURNAL.PONE.0056522
Hudlicka O (2011) Microcirculation in skeletal muscle. Muscles Ligaments Tendons J 1:3
Camacho-Cardenosa M, Camacho-Cardenosa A, Kemp J et al (2021) Haematological responses to repeated sprints in hypoxia across different sporting modalities. Res Sports Med 30:529–539. https://doi.org/10.1080/15438627.2021.1917403
Brechbuhl C, Brocherie F, Millet GP, Schmitt L (2018) Effects of repeated-sprint training in hypoxia on tennis-specific performance in well-trained players. Sports Med Int Open 02:E123–E132. https://doi.org/10.1055/A-0719-4797
Raberin A, Elmer J, Willis SJ, Richard T, Vernillo G, Iaia M, Millet GP (2022) The oxidative-glycolytic balance influenced by sprint duration is key during repeated sprint in hypoxia. Med Sci Sports Exerc. https://doi.org/10.1249/MSS.0000000000003042
Etxebarria N, Ingham SA, Ferguson RA et al (2019) Sprinting after having sprinted: prior high-intensity stochastic cycling impairs the winning strike for gold. Front Physiol 10:100. https://doi.org/10.3389/fphys.2019.00100
Faiss R, Rapillard A (2020) Repeated sprint training in hypoxia: case report of performance benefits in a professional cyclist. Front Sports Act Living 2:35. https://doi.org/10.3389/fspor.2020.00035
Vasquez-Bonilla AA, Camacho-Cardeñosa A, Timón R, Martínez-Guardado I, Camacho-Cardeñosa M, Olcina G (2021) Muscle oxygen desaturation and re-saturation capacity limits in repeated sprint ability performance in women soccer players: a new physiological interpretation. Int J Environ Res Public Health 18:3484. https://doi.org/10.3390/ijerph18073484
Vasquez-Bonilla AA, Rojas-Valverde D, Gonzalez-Custodio A et al (2021) Tent vs. mask-on acute effects during repeated-sprint training in normobaric hypoxia and normoxia. J Clin Med 10:4879. https://doi.org/10.3390/JCM10214879
Zhao J, Lorenzo S, An N et al (2013) Effects of heat and different humidity levels on aerobic and anaerobic exercise performance in athletes. J Exerc Sci Fit 11:35–41. https://doi.org/10.1016/J.JESF.2013.04.002
Liang M, Chen TI, Lee JN-Y et al (2013) Effect of short-term heat acclimation on endurance time and skin blood flow in trained athletes. Open Access J Sports Med 4:161. https://doi.org/10.2147/OAJSM.S45024
Nair B (2019) Clinical trial designs. Indian Dermatol Online J 10:193–201. https://doi.org/10.4103/idoj.IDOJ_475_18
Efird J (2010) Blocked randomization with randomly selected block sizes. Int J Environ Res Public Health 8(1):15–20. https://doi.org/10.3390/IJERPH8010015
Brocherie F, Millet GP, D’Hulst G et al (2018) Repeated maximal-intensity hypoxic exercise superimposed to hypoxic residence boosts skeletal muscle transcriptional responses in elite team-sport athletes. Acta Physiol 222:e12851. https://doi.org/10.1111/APHA.12851
Arezzolo D, Coffey VG, Byrne NM, Doering TM (2020) Effects of eight interval training sessions in hypoxia on anaerobic, aerobic, and high intensity work capacity in endurance cyclists. High Alt Med Biol 21:370–377. https://doi.org/10.1089/HAM.2020.0066
Jeukendrup AE, Craig NP, Hawley JA (2000) The bioenergetics of world class cycling. J Sci Med Sport 3:414–433. https://doi.org/10.1016/S1440-2440(00)80008-0
Glaister M, Howatson G, Pattison JR, McInnes G (2008) The reliability and validity of fatigue measures during multiple-sprint work: an issue revisited. J Strength Cond Res 22:1597–1601. https://doi.org/10.1519/JSC.0b013e318181ab80
Romer LM, Haverkamp HC, Lovering AT et al (2006) Effect of exercise-induced arterial hypoxemia on quadriceps muscle fatigue in healthy humans. Am J Physiol Regul Integr Comp Physiol 290:365–375. https://doi.org/10.1152/AJPREGU.00332.2005
Crum EM, O’Connor WJ, Van Loo L et al (2017) Validity and reliability of the moxy oxygen monitor during incremental cycling exercise. Eur J Sport Sci 17:1037–1043. https://doi.org/10.1080/17461391.2017.1330899
Shibuya K, Tanaka J (2003) Skeletal muscle oxygenation during incremental exercise. Arch Physiol Biochem 111:475–478. https://doi.org/10.3109/13813450312331342355
Rodriguez RF, Townsend NE, Aughey RJ, Billaut F (2018) Influence of averaging method on muscle deoxygenation interpretation during repeated-sprint exercise. Scand J Med Sci Sports. https://doi.org/10.1111/sms.13238
Verdel N, Podlogar T, Ciuha U et al (2021) Reliability and validity of the core sensor to assess core body temperature during cycling exercise. Sensors 21:5932. https://doi.org/10.3390/S21175932
Cohen J. (1988) Statistical power analysis for the behavioural science (2nd edition) in: statistical power anaylsis for the behavioural Science (2nd Edition)
Weir JP (2005) Quantifying test-retest reliability using the intraclass correlation coefficient and the sem. J Strength Cond Res 19:231–240. https://doi.org/10.1519/00124278-200502000-00038
Fitzpatrick JF, Akenhead R, Russell M et al (2019) Sensitivity and reproducibility of a fatigue response in elite youth football players. Sci Med Footb 3(3):214–220
Millet GP, Girard O (2017) Editorial: high-intensity exercise in hypoxia: beneficial aspects and potential drawbacks. Front Physiol 8:1017. https://doi.org/10.3389/FPHYS.2017.01017/BIBTEX
Montero D, Lundby C (2017) No improved performance with repeated-sprint training in hypoxia vs. normoxia: a double-blind and crossover study. Int J Sports Physiol Perform 12:161–167. https://doi.org/10.1123/IJSPP.2015-0691
Umberger BR, Gerritsen KGM, Martin PE (2006) Muscle fiber type effects on energetically optimal cadences in cycling. J Biomech 39:1472–1479. https://doi.org/10.1016/J.JBIOMECH.2005.03.025
Kasai N, Mizuno S, Ishimoto S et al (2015) Effect of training in hypoxia on repeated sprint performance in female athletes. Springerplus 4:1–7. https://doi.org/10.1186/S40064-015-1041-4/TABLES/2
Allen SV, Hopkins WG (2015) Age of peak competitive performance of elite athletes: a systematic review. Sports Med 45:1431–1441. https://doi.org/10.1007/S40279-015-0354-3/FIGURES/2
Bejder J, Andersen AB, Buchardt R et al (2017) Endurance, aerobic high-intensity, and repeated sprint cycling performance is unaffected by normobaric “live high-train low”: a double-blind placebo-controlled cross-over study. Eur J Appl Physiol 117:979–988. https://doi.org/10.1007/S00421-017-3586-0/FIGURES/5
Morrison J, McLellan C, Minahan C (2015) A clustered repeated-sprint running protocol for team-sport athletes performed in normobaric hypoxia. J Sports Sci Med 14:857
McGawley K, Bishop DJ (2015) Oxygen uptake during repeated-sprint exercise. J Sci Med Sport. https://doi.org/10.1016/j.jsams.2014.02.002
Børsheim E, Bahr R (2003) Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Medicine
Wolff CB (2008) Normal cardiac output oxygen delivery and oxygen extraction. Adv Exp Med Biol 599:169–182. https://doi.org/10.1007/978-0-387-71764-7_23/COVER
Gregory G, Ae D, Gregoire G et al (2005) Relationship between oxygen uptake kinetics and performance in repeated running sprints. Eur J Appl Physiol 95(1):27–34. https://doi.org/10.1007/S00421-005-1382-8
Faiss R, Willis S, Born DP et al (2015) Repeated double-poling sprint training in hypoxia by competitive cross-country skiers. Med Sci Sports Exerc 47:809–817. https://doi.org/10.1249/MSS.0000000000000464
Bastien Racinais SÉ, Bishop D, Denis R et al (2007) Muscle deoxygenation and neural drive to the muscle during repeated sprint cycling. Med Sci Sports Exerc 39:268–274. https://doi.org/10.1249/01.mss.0000251775.46460.cb
Pramkratok W, Songsupap T, Yimlamai T (2022) Repeated sprint training under hypoxia improves aerobic performance and repeated sprint ability by enhancing muscle deoxygenation and markers of angiogenesis in rugby sevens. Eur J Appl Physiol 122:611–622. https://doi.org/10.1007/S00421-021-04861-8/FIGURES/3
Stroka DM, Burkhardt T, Desbaillets I et al (2001) HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 15:2445–2453. https://doi.org/10.1096/fj.01-0125com
Wagenmakers AJM, Strauss JA, Shepherd SO et al (2016) The journal of physiology increased muscle blood supply and transendothelial nutrient and insulin transport induced by food intake and exercise: effect of obesity and ageing. J Physiol 594:2207–2222. https://doi.org/10.1113/jphysiol.2014.284513
Thomas GD, Victor RG (1998) Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. https://doi.org/10.1111/j.1469-7793.1998.817bv.x
Vasquez-bonilla AA, Rojas-Valverde D, Timon R, Olcina G (2022) Influence of fat percentage on muscle oxygen uptake and metabolic power during repeated-sprint ability of footballers. Apunts Sports Med 57:100395. https://doi.org/10.1016/J.APUNSM.2022.100395
Billaut F, Kerris JP, Rodriguez RF et al (2013) Interaction of central and peripheral factors during repeated sprints at different levels of arterial O2 saturation. PLoS ONE 8:e77297. https://doi.org/10.1371/JOURNAL.PONE.0077297
Brocherie F, Girard O, Faiss R, Millet GP (2015) High-intensity intermittent training in hypoxia: a double-blinded, placebo-controlled field study in youth football players. J Strength Cond Res 29:226–237. https://doi.org/10.1519/JSC.0000000000000590
Brooks GA (2007) Lactate: link between glycolytic and oxidative metabolism. Sports Med 37:341–343. https://doi.org/10.2165/00007256-200737040-00017/METRICS
Brooks GA (2002) Lactate shuttles in nature. Biochem Soc Trans 30:258–264. https://doi.org/10.1042/BST0300258
Pellerin L, Pellegri G, Bittar PG et al (1998) Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci 20:291–299. https://doi.org/10.1159/000017324
Ferguson BS, Rogatzki MJ, Goodwin ML et al (2018) Lactate metabolism historical context prior misinterpretaions, and current understanding. Eur J Appl Physiol 118(4):691–728
Macdougall JD, Hicks AL, Macdonald JR et al (1998) Muscle performance and enzymatic adaptations to sprint interval training. J Appl Physiol 84:2138–2142. https://doi.org/10.1152/JAPPL.1998.84.6.2138
Puype J, Van Proeyen K, Raymackers J et al (2013) Sprint interval training in hypoxia stimulates glycolytic enzyme activity. Med Sci Sports Exerc. https://doi.org/10.1249/MSS.0b013e31829734ae
Zoll J, Ponsot E, Dufour S et al (2006) Exercise training in normobaric hypoxia in endurance runners. III. muscular adjustments of selected gene transcripts. J Appl Physiol 100:1258–1266. https://doi.org/10.1152/JAPPLPHYSIOL.00359
Wenger RH (2002) Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 16:1151–1162. https://doi.org/10.1096/FJ.01-0944REV
Terrados N, Jansson E, Sylven C, Kaijser L (1990) Is hypoxia a stimulus for synthesis of oxidative enzymes and myoglobin? J Appl Physiol 68:2369–2372. https://doi.org/10.1152/JAPPL.1990.68.6.2369
Vogt M, Puntschart A, Geiser J et al (2001) Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J Appl Physiol 91:173–182. https://doi.org/10.1152/JAPPL.2001.91.1.173/ASSET/IMAGES/LARGE/DG0710798003.JPEG
Girard O, Brocherie F, Bishop DJ (2015) Sprint performance under heat stress: a review. Scand J Med Sci Sports 25:79–89. https://doi.org/10.1111/SMS.12437
Dennis MC, Goods PSR, Binnie MJ et al (2022) Repeated-sprint training in heat and hypoxia: acute responses to manipulating exercise-to-rest ratio. Eur J Sport Sci. https://doi.org/10.1080/17461391.2022.2085631
Dennis MC, Goods PSR, Binnie MJ et al (2021) Increased air temperature during repeated-sprint training in hypoxia amplifies changes in muscle oxygenation without decreasing cycling performance. Eur J Sport Sci 10(1080/17461391):2003868. https://doi.org/10.1080/17461391.2021.2003868
Araneda OF, Carbonell T, Tuesta M (2016) Update on the mechanisms of pulmonary inflammation and oxidative imbalance induced by exercise. Oxid Med Cell Longev. https://doi.org/10.1155/2016/4868536
Anderson SD, Kippelen P (2005) Exercise-induced bronchoconstriction: pathogenesis. Curr Allergy Asthma Rep 5:116–122. https://doi.org/10.1007/S11882-005-0084-Y/METRICS
Parsons JP, Hallstrand TS, Mastronarde JG et al (2013) An official american thoracic society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med 187:1016–1027. https://doi.org/10.1164/RCCM.201303-0437ST
Wolkoff P (2018) Indoor air humidity air quality and health—an overview. Int J Hyg Environ Health 221:376–390. https://doi.org/10.1016/J.IJHEH.2018.01.015
Contreras-Briceño F, Espinosa-Ramirez M, Viscor G, Araneda OF (2020) Humidity prevents the exercise-induced formation of hydrogen peroxide and nitrite in exhaled breath condensate in recreational cyclists. Eur J Appl Physiol 120:2339–2348. https://doi.org/10.1007/S00421-020-04456-9
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study has been supported by Junta de Extremadura (Spain) with funding from the European Regional Development Fund and and the postdoctoral contract Margarita Salas reference MS-35 (University of Extremadura) from the program of requalification of the Spanish University System (Spanish Ministry of universities) financed by the European Union-NextGenerationEU.
Author information
Authors and Affiliations
Contributions
Author Contributions: Research concept and study design, A.V. and G.O.; literature review, G.O., D.R-V., R.T. and J.M.; data collection, A.V. and J.M.; data analysis and interpretation, A.V., D.R-V, R.T., and G-O.; Statical analyses, A.V. and J.M.; writing of the manuscript. A.V., D.R-V, and R.T.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University of Extremadura (code register: 174/2020).
Informed consent
Written informed consent is obtained from all participants in this study after giving complete information about the hypoxia training and measurements, and its possible effects and complications.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vasquez-Bonilla, A.A., Rojas-Valverde, D., Feliu-Ilvonen, J.M. et al. Mask vs. tent: effect of hypoxia method on repeated sprint ability and physiological parameters in cyclists. Sport Sci Health (2024). https://doi.org/10.1007/s11332-024-01218-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11332-024-01218-4