Abstract
Background
Sedentary lifestyle habits are directly related to the increase in the production of reactive oxygen species, to provide a chronic state of oxidative stress. Furthermore, the purinergic system is involved in extracellular signaling and thus influences pathological and physiological processes. Thus, physical exercise is responsible for triggering molecular and tissue changes, to condition the body’s pathophysiological processes, being often suggested as a form of non-pharmacological treatment in different health conditions.
Aim
To evaluate the effect of physical exercise on markers of oxidative stress and the purinergic system in young adults.
Method
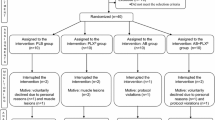
Sixteen women students, aged between 20 and 40 years, were submitted to the physical exercise protocol, both aerobic and resistance (concurrent training) during the period of eight weeks, making a total of 20 h of training.
Results
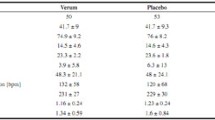
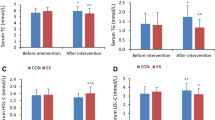
Our findings showed that physical exercise was responsible for increasing the hydrolysis of adenosine diphosphate (ADP) (p = 0.001) and adenosine deaminase (ADA) (p = 0.041) in platelets, indicating an increase in ecto-nucleoside triphosphate diphosphohydrolase (ecto-NTPDase) after eight weeks of physical exercise. Furthermore, there was an increase in the concentration of thiobarbituric acid reactive substances (TBARS) (p = 0.012) and protein thiols (PSH) (p = 0.004) after one week of exercise. Furthermore, it can be considered that resistance exercise can stimulate the antioxidant defense mechanisms as well as promote an anti-inflammatory and anti-platelet aggregation state.
Conclusion
In conclusion, physical exercise alters both the mechanisms of oxidative stress and the purinergic system, to have the potential to attenuate inflammatory mechanisms.




Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
World health organization. Global recommendations on physical activity for health. https://www.who.int/dietphysicalactivity/factsheet_recommendations/en/ (2010). Accessed 5 june 2020
Dempsey PC, Owen N, Biddle SJH, Dustan DW (2014) Managing sedentary behavior to reduce the risk of diabetes and cardiovascular disease. Curr Diab Rep 14:522. https://doi.org/10.1007/s11892-014-0522-0
Egan B, Zierath JR (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17:162–184. https://doi.org/10.1016/j.cmet.2012.12.012
Mathur N, Pedersen BK (2008) Exercise as a mean to control low-grade systemic inflammation. Mediat Inflamm 2008:1–6. https://doi.org/10.1155/2008/109502
Nunes RB, Alves JP, Kessler LP, Lago PD (2013) Aerobic exercise improves the inflammatory profile correlated with cardiac remodeling and function in chronic heart failure rats. Clinics (São Paulo) 68:876–882. https://doi.org/10.6061/clinics/2013(06)24
Greaney JL, Wenner MM, Farquhar WB (2015) Exaggerated increases in blood pressure during isometric muscle contraction in hypertension: role for purinergic receptors. Auton Neurosci 188:51–57. https://doi.org/10.1016/j.autneu.2014.12.003
Junger WG (2011) Immune cell regulation by autocrine purinergic signaling. Nat Rev Immunol 11:201–212. https://doi.org/10.1038/nri2938
Burnstock G (2007) Purine and pyrimidine receptors. Cell Mol Life Sci 64:1471–1483. https://doi.org/10.1007/s00018-007-6497-0
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067. https://doi.org/10.1152/physrev.00015.2002
Böyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 97:77–89
Pilla C, Emanuelli T, Frassetto SS, Battastini AM, Dias RD, Sarkis JJ (1996) ATP diphosphohydrolase activity (Apyrase, EC 3.6.1.5.) in human blood platelets. Platelets 7:225–230. https://doi.org/10.3109/09537109609023582
Lunkes GI, Lunkes D, Stefanello F, Morsch A, Morsch VM, Mazzanti CM, Schetinger MRC (2003) Enzymes that hydrolyze adenine nucleotides in diabetes and associated pathologies. Thromb Res 109:189–194. https://doi.org/10.1016/s0049-3848(03)00178-6
Jentzsch AM, Bachmann H, Fürst P, Biesalski HK (1996) Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med 20:251–256. https://doi.org/10.1016/0891-5849(95)02043-8
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:768–774. https://doi.org/10.1016/0003-9861(59)90090-6
Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T (1983) Assay Method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem 132:345–352. https://doi.org/10.1016/0003-2697(83)90019-2
Giusti G, Galanti B (1984) Adenosine deaminase: colorimetric method. Methods Enzym Anal 4:315–323
Bradford MA (1976) Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principie ofprotein-dyebinding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Moritz CEJ, Teixeira BC, Rockenbach L, Reischak-Oliveira A, Casali EA, Battastini AMO (2017) Altered extracellular ATP, ADP, and AMP hydrolysis in blood serum of sedentary individuals after an acute, aerobic, moderate exercise session. Clin Trial 426:55–63. https://doi.org/10.1007/s11010-016-2880-1
Bours MJL, Swennen ELR, Di Virgilio F, Cronstein BN, Dagnelie PC (2006) Adenosine 5’-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 112:358–404. https://doi.org/10.1016/j.pharmthera.2005.04.013
Cardoso AM, Abdalla FH, Bagatini MD, Martins CC, Zanini D, Schmatz R, Jaques JA, Leal DBR, Morsch VM, Schetinger MRC (2015) Swimming training prevents alterations in ecto-NTPDase and adenosine deaminase activities in lymphocytes from Nω-nitro-L-arginine methyl ester hydrochloride induced hypertension rats. J Hypertens 33:763–772. https://doi.org/10.1097/hjh.0000000000000468
Gleeson M, Bispo NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MAV (2011) The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11:607–615. https://doi.org/10.1038/nri3041
Fürstenau CR, Trentin DDS, Barreto-chaves MLM, Sarkis JJF (2006) Ecto-nucleotide pyrophosphatase/phosphodiesterase as part of a multiple system for nucleotide hydrolysis by platelets from rats: kinetic characterization and biochemical properties. Platelets 17:84–91. https://doi.org/10.1080/09537100500246641
Podgorska K, Derkacz A, Szahidewicz-Krupska E, Jasiczek J, Dobrowolski P, Radziwon-Balicka A, Skomro R, Szuba A, Mazur G, Doroszko A (2017) Effect of regular aerobic activity in young healthy athletes on profile of endothelial function and platelet activity. Biomed Res Int 2017:1–9
Uppu RM, Woods D, Nl Parinandi (2020) Measurement of Oxidative Stress Status in Human Populations: a critical need for a metabolomic profiling. Measuring Oxidants And Oxidative Stress In Biological Systems. Springer, Cham, pp 123–131
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:1–31
Schuch FB, Vasconcelos-Moreno MP, Borowsky C, Zimmermann AB, Wollenhaupt-Aguiar B, Ferrari P, Fleck MPA (2014) The effects of exercise on oxidative stress (TBARS) and BDNF in severely depressed inpatients. Eur Arch Of Psychiatry Clin Neurosci 264:605–613. https://doi.org/10.1007/s00406-014-0489-5
Farinha JB, Steckling FM, Stefanello ST, Cardoso MS, Nunes LS, Barcelos RP, Duarte T, Kretzmann NA, Mota CB, Bresciani G, Moresco RN, Duarte MMMF, Santos DL, Soares FAA (2015) Response of oxidative stress and inflammatory biomarkers to a 12-week aerobic exercise training in women with metabolic syndrome. Sports Med Open 1:19. https://doi.org/10.1186/s40798-015-0011-2
Rio D, Stewart AJ, Pellegrini NA (2005) A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 15:316–328. https://doi.org/10.1016/j.numecd.2005.05.003
Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, Burt AD, Dia CP (2005) Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut 54:987–993
Papac-Milicevic N, Busch CJL, Binder CJ (2016) malondialdehyde epitopes as targets of immunity and the implications for atherosclerosis. Adv Immunol 131:1–59. https://doi.org/10.1016/bs.ai.2016.02.001
Zagol-Ikapite I, Sosa IR, Oram D, Judd A, Amarnath K, Amarnath V, Stec D, Oates JA, Boutaud O (2015) Modification of platelet proteins by malondialdehyde: prevention by dicarbonyl scavengers. J Lipid Res 56:2196–2205
Aratani Y (2018) Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys 640:47–52. https://doi.org/10.1016/j.abb.2018.01.004
Yang Y, Guan X (2017) Non-protein thiol imaging and quantification in live cells with a novel benzofurazan sulfide triphenylphosphonium fluorogenic compound. Anal Bioanal Chem 409:3417–3427. https://doi.org/10.1007/s00216-017-0285-y
Ulrich K, Jakob U (2019) The role of thiols in antioxidant systems. Free Radic Biol Med 140:14–27. https://doi.org/10.1016/j.freeradbiomed.2019.05.035
Rossi R, Giustarini D, Milzani A, Dalle-Donne I (2008) Cysteinylation and homocysteinylation of plasma protein thiols during ageing of healthy human beings. J Cell Mol Med 13:3131–3140. https://doi.org/10.1111/j.1582-4934.2008.00417.x
Zinellu A, Sotgia S, Caria MA, Tangianu F, Casu G, Deiana L, Carru C (2006) Effect of acute exercise on low molecular weight thiols in plasma. ScandJ Med Sci Sports 17:452–456. https://doi.org/10.1111/j.1600-0838.2006.00542.x
Laaksonen DE, Atalay M, Niskanen L, Uusitupa M, Hänninen O, Sen CK (1999) Blood glutathione homeostasis as a determinant of resting and exercise-induced oxidative stress in young men. Redox Rep 4:53–59. https://doi.org/10.1179/135100099101534648
Zinellu A, Fois AG, Sotgia S, Zinellu E, Bifulco F, Pintus G, Mangoni AA, Carru C, Pirina P (2016) Plasma protein thiols: an early marker of oxidative stress in asthma and chronic obstructive pulmonary disease. Eur J Clin Invest 46:181–188. https://doi.org/10.1111/eci.12582
Picklo MJ, Idso JP, Jackson MI (2013) S-Glutathionylation of Hepatic and Visceral Adipose Proteins Decreases in Obese Rats. Obesity 21:297–305
Lepedda AJ, Formato M (2020) oxidative modifications in advanced atherosclerotic plaques: a focus on in situ protein sulfhydryl group oxidation. Oxid Med Cell Longev 2020:1–7
Patsoukis N, Papapostolou I, Zervoudakis G, Georgiou CD, Matsokis NA, Panagopoulos NT (2004) Thiol redox state and oxidative stress in midbrain and striatum of weaver mutant mice, a genetic model of nigrostriatal dopamine deficiency. Neurosci Lett 376:24–28. https://doi.org/10.1016/j.neulet.2004.11.019
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: MRB, FBG and DTReS; Methodology: ECS, FM, BSRB and MDB; Formal analysis and investigation: MRB and FBH; Writing–preparation of the original sketch: MRB and ECS; Writing–revision and editing: MRB and DTReS.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declares there is no conflict of interest.
Ethical approval
All procedures were approved by the Research Ethics Committee (CEP) of the Institute of Cardiology of Rio Grande do Sul/University Foundation of Cardiology and was registered through Brazil Platform under CAAE: 85407018.3.0000.5333. Participants were informed about the purpose of the research and about the methods used for data collection and intervention, as well as their evaluation. Finally, all the requirements of the law were followed to maintain the respect, dignity and freedom of the participants.
Informed consent
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bizuti, M.R., de Camargo Schwede, E., Haag, F.B. et al. Concurrent training is able to increase the activity of adenosine deaminase in platelets in young women. Sport Sci Health 19, 849–859 (2023). https://doi.org/10.1007/s11332-022-00970-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-022-00970-9