Abstract
Background
Studies about antioxidant supplementation and exercises combined, especially at hepatic liver tissue, are rare and still controversial. In this study, we aimed to evaluate if the association between a recognized antioxidant compound—Diphenyl Diselenide ([(PhSe)2])—and training can reduce homogenate liver and liver mitochondria oxidative stress in old rats.
Methods
Old male Wistar rats were divided into four groups (six animals per group): old-sedentary, old-sedentary [(PhSe)2] supplemented, old-trained, and old-trained [(PhSe)2] supplemented. Trained groups were submitted to swimming training sessions (3% of body weight, 20 min/day during 4 weeks); animals were fed daily with standard feed or standard feed supplemented with 1 ppm of [(PhSe)2] during 4 weeks.
Results
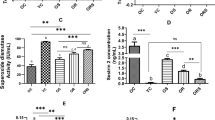
Trained and trained + [(PhSe)2] groups decreased reactive oxygen species (ROS) generation, while only the trained group reduces GSSG production and increased GSH/GSSG ratio when compared to trained + [(PhSe)2]. Mitochondrial ROS production was elevated in control sedentary group, but only swimming training prevented its elevation. However, MnSOD activity was found elevated at trained + [(PhSe)2] rats when compared to the trained and [(PhSe)2] supplementation groups. Mitochondrial Δψm in trained + [(PhSe)2] was decreased compared to trained group, while ratio (III/IV states) was increased when compared to control sedentary.
Conclusions
We conclude that the combination of [(PhSe)2] and swimming training did not manifest synergic effect since it does not prevent the aging-induced hepatic oxidative stress generation, but blunted the induced-exercise adaptations, including at mitochondrial mechanisms.




Similar content being viewed by others
Abbreviations
- [(PhSe)2]:
-
Diphenyl diselenide
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- MnSOD:
-
Manganese superoxide dismutase
- Δψm :
-
Mitochondrial transmembrane electrical potential
- H2DCF-DA:
-
Reduced dichlorofluorescein diacetate
- DCF:
-
Oxidized dichlorofluorescein
- OPT:
-
O-Phthalaldehyde
- KCN:
-
Potassium cyanide
References
Alberti KGMM, Zimmet P, Shaw J (2007) International diabetes federation: a consensus on Type 2 diabetes prevention. Diabet Med 24:451–463. https://doi.org/10.1111/j.1464-5491.2007.02157.x
Warburton DER, Nicol CW, Bredin SSD (2006) Health benefits of physical activity: the evidence. CMAJ 174:801–809. https://doi.org/10.1503/cmaj.051351
Viña J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC (2012) Exercise acts as a drug; The pharmacological benefits of exercise. Br J Pharmacol 167:1–12
Steinbacher P, Eckl P (2015) Impact of oxidative stress on exercising skeletal muscle. Biomolecules 5:356–377
Gomez-Cabrera MC, Domenech E, Viña J (2008) Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44:126–131. https://doi.org/10.1016/j.freeradbiomed.2007.02.001
Rasmussen UF, Krustrup P, Kjær M, Rasmussen HN (2003) Experimental evidence against the mitochondrial theory of aging A study of isolated human skeletal muscle mitochondria. Exp Gerontol 38:877–886. https://doi.org/10.1016/S0531-5565(03)00092-5
Ji LL (1993) Antioxidant enzyme response to exercise and aging. Med Sci Sports Exerc 25:225–231
Navarro A, Boveris A (2007) The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292:C670–C686. https://doi.org/10.1152/ajpcell.00213.2006
Houtkooper RH, Argmann C, Houten SM, Cantó C, Jeninga EH, Andreux PA, Thomas C, Doenlen R, Schoonjans K, Auwerx J (2011) The metabolic footprint of aging in mice. Sci Rep. https://doi.org/10.1038/srep00134
Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C (2009) Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8:18–30
Arnér ESJ (2009) Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim Biophys Acta Gen Subj 1790:495–526
Nogueira CW, Rocha JBT (2011) Toxicology and pharmacology of selenium: emphasis on synthetic organoselenium compounds. Arch Toxicol 85:1313–1359
Brenneisen P, Steinbrenner H, Sies H (2005) Selenium, oxidative stress, and health aspects. Mol Aspects Med 26:256–267
Parnham M, Sies H (2000) Ebselen: prospective therapy for cerebral ischaemia. Expert Opin Investig Drugs 9:607–619. https://doi.org/10.1517/13543784.9.3.607
Ryan-Harshman M, Aldoori W (2005) The relevance of selenium to immunity, cancer, and infectious/inflammatory diseases. Can J Diet Pract Res 66:98–102
Luchese C, Pinton S, Nogueira CW (2009) Brain and lungs of rats are differently affected by cigarette smoke exposure: antioxidant effect of an organoselenium compound. Pharmacol Res 59:194–201. https://doi.org/10.1016/j.phrs.2008.11.006
Prigol M, Schumacher RF, WayneNogueira C, Zeni G (2009) Convulsant effect of diphenyl diselenide in rats and mice and its relationship to plasma levels. Toxicol Lett 189:35–39. https://doi.org/10.1016/j.toxlet.2009.04.026
Carvalho NR, Da Rosa EF, Da Silva MH, Tassi CC, Corte CLD, Carbajo-Pescador S, Mauriz JL, González-Gallego J, Soares FA (2013) New therapeutic approach: diphenyl diselenide reduces mitochondrial dysfunction in acetaminophen-induced acute liver failure. PLoS One. https://doi.org/10.1371/journal.pone.0081961
Nogueira CW, Rocha JBT (2010) Diphenyl diselenide a janus-faced molecule. J Braz Chem, Soc
Rosa RM, Roesler R, Braga AL, Saffi J, Henriques JAP (2007) Pharmacology and toxicology of diphenyl diselenide in several biological models. Braz J Med Biol Res 40:1287–1304
Leite MR, Cechella JL, Mantovani AC, Duarte MMMF, Nogueira CW, Zeni G (2015) Swimming exercise and diphenyl diselenide-supplemented diet affect the serum levels of pro- and anti-inflammatory cytokines differently depending on the age of rats. Cytokine. https://doi.org/10.1016/j.cyto.2014.09.006
Heck SO, Fulco BCW, Quines CB, Oliveira CES, Leite MR, Cechella JL, Nogueira CW (2017) Combined therapy with swimming exercise and a diet supplemented with diphenyl diselenide is effective against. J Cell Biochem. https://doi.org/10.1002/jcb.25819
Paulmier C (1986) Selenium reagents and intermediates in organic synthesis. Pergamon Press. https://doi.org/10.1002/ange.19881000236
de Bem AF, Portella RDL, Colpo E, Duarte MMMF, Frediane A, Taube PS, Nogueira CW, Farina M, da Silva EL, Teixeira Rocha JB (2009) Diphenyl diselenide decreases serum levels of total cholesterol and tissue oxidative stress in cholesterol-fed rabbits. Basic Clin Pharmacol Toxicol 105:17–23. https://doi.org/10.1111/j.1742-7843.2009.00414.x
de Bem AF, de Lima Portella R, Perottoni J, Becker E, Bohrer D, Paixão MW, Nogueira CW, Zeni G, Rocha JBT (2006) Changes in biochemical parameters in rabbits blood after oral exposure to diphenyl diselenide for long periods. Chem Biol Interact. https://doi.org/10.1016/j.cbi.2006.04.005
De Bem AF, Portella RDL, Farina M, Perottoni J, Paixão MW, Nogueira CW, Rocha JBT (2007) Low toxicity of diphenyl diselenide in rabbits: a long-term study. Basic Clin Pharmacol Toxicol. https://doi.org/10.1111/j.1742-7843.2007.00073.x
Ravi Kiran T, Subramanyam MVV, Asha Devi S (2004) Swim exercise training and adaptations in the antioxidant defense system of myocardium of old rats: relationship to swim intensity and duration. Comp Biochem Physiol B Biochem Mol Biol 137:187–196. https://doi.org/10.1016/j.cbpc.2003.11.002
Bhattacharya SK, Thakar JH, Johnson PL, Shanklin DR (1991) Isolation of skeletal muscle mitochondria from hamsters using an lonic medium containing ethylenediarninetetraacetic acid and nagarse. Anal Biochem 192:344–349. https://doi.org/10.1016/0003-2697(91)90546-6
Kruglov AG, Teplova VV, Saris NEL (2007) The effect of the lipophilic cation lucigenin on mitochondria depends on the site of its reduction. Biochem Pharmacol 74:545–556. https://doi.org/10.1016/j.bcp.2007.05.012
Myhre O, Andersen JM, Aarnes H, Fonnum F (2003) Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65:1575–1582
García-Ruiz C, Colell A, Marí M, Morales A, Fernández-Checa JC (1997) Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species: role of mitochondrial glutathione. J Biol Chem. https://doi.org/10.1074/jbc.272.17.11369
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226. https://doi.org/10.1016/0003-2697(76)90326-2
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(3170–3175):4623845
Geller BL, Winge DR (1984) Subcellular distribution of superoxide dismutases in rat liver. Methods Enzymol 105:105–114. https://doi.org/10.1016/S0076-6879(84)05014-X
Åkerman KEO, Wikström MKF (1976) Safranine as a probe of the mitochondrial membrane potential. FEBS Lett 68:191–197. https://doi.org/10.1016/0014-5793(76)80434-6
Da-Silva WS, Gómez-Puyou A, De Gómez-Puyou MT, Moreno-Sanchez R, De Felice FG, De Meis L, Oliveira MF, Galina A (2004) Mitochondrial bound hexokinase activity as a preventive antioxidant defense. Steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J Biol Chem 279:39846–39855. https://doi.org/10.1074/jbc.M403835200
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ji LL (2015) Redox signaling in skeletal muscle: role of aging and exercise. Adv Physiol Educ 39:352–359. https://doi.org/10.1152/advan.00106.2014
Safwat MH, El-Sawalhi MM, Mausouf MN, Shaheen AA (2014) Ozone ameliorates age-related oxidative stress changes in rat liver and kidney: effects of pre- and post-ageing administration. Biochem 79:450–458. https://doi.org/10.1134/S0006297914050095
Kan H, Hu W, Wang Y, Wu W, Yin Y, Liang Y, Wang C, Huang D, Li W (2015) NADPH oxidase-derived production of reactive oxygen species is involved in learning and memory impairments in 16-month-old female rats. Mol Med Rep 12:4546–4553. https://doi.org/10.3892/mmr.2015.3894
Lima FD, Stamm DN, Della-Pace ID, Dobrachinski F, de Carvalho NR, Royes LFF, Soares FA, Rocha JB, González-Gallego J, Bresciani G (2013) Swimming training induces liver mitochondrial adaptations to oxidative stress in rats submitted to repeated exhaustive swimming bouts. PLoS One. https://doi.org/10.1371/journal.pone.0055668
Santos-Alves E, Marques-Aleixo I, Coxito P, Balça MM, Rizo-Roca D, Rocha-Rodrigues S, Martins S, Torrella JR, Oliveira PJ, Moreno AJ, Magalhães J, Ascensão A (2014) Exercise mitigates diclofenac-induced liver mitochondrial dysfunction. Eur J Clin Invest 44:668–677. https://doi.org/10.1111/eci.12285
Barcelos RP, Souza MA, Amaral GP, Stefanello ST, Bresciani G, Fighera MR, Soares FAA, Barbosa NV (2014) Caffeine supplementation modulates oxidative stress markers in the liver of trained rats. Life Sci 96:40–45. https://doi.org/10.1016/j.lfs.2013.12.002
Radák Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, Goto S (2004) Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J 18:749–750. https://doi.org/10.1096/fj.03-0509fje
Radak Z, Taylor AW, Ohno H, Goto S (2001) Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev 7:90–107
Merry TL, Ristow M (2016) Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol. https://doi.org/10.1113/JP270654
Barcelos RP, Bresciani G, Rodriguez-Miguelez P, Cuevas MJ, Soares FAA, Barbosa NV, González-Gallego J (2016) Diclofenac pretreatment effects on the toll-like receptor 4/nuclear factor kappa B-mediated inflammatory response to eccentric exercise in rat liver. Life Sci. https://doi.org/10.1016/j.lfs.2016.02.006
Puntel RL, Roos DH, Folmer V, Nogueira CW, Galina A, Aschner M, Rocha JBT (2010) Mitochondrial dysfunction induced by different organochalchogens is mediated by thiol oxidation and is not dependent of the classical mitochondrial permeability transition pore opening. Toxicol Sci 117:133–143. https://doi.org/10.1093/toxsci/kfq185
Bhatti JS, Bhatti GK, Reddy PH (2017) Mitochondrial dysfunction and oxidative stress in metabolic disorders—a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis 1863:1066–1077
Puntel RL, Roos DH, Seeger RL, Rocha JBT (2013) Mitochondrial electron transfer chain complexes inhibition by different organochalcogens. Toxicol Vitr 27:59–70. https://doi.org/10.1016/j.tiv.2012.10.011
Nogueira CW, Zeni G, Rocha JBT (2004) Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev. https://doi.org/10.1021/cr0406559
de Jong N, Gibson RS, Thomson CD, Ferguson EL, McKenzie JE, Green TJ, Horwath CC (2001) Selenium and zinc status are suboptimal in a sample of older New Zealand women in a community-based study. J Nutr 131:2677–2684
Shen CL, Song W, Pence BC (2001) Interactions of selenium compounds with other antioxidants in DNA damage and apoptosis in human normal keratinocytes. Cancer Epidemiol Biomarkers Prev 10:385–390
Morin D, Zini R, Ligeret H, Neckameyer W, Labidalle S, Tillement JP (2003) Dual effect of ebselen on mitochondrial permeability transition. Biochem Pharmacol 65:1643–1651. https://doi.org/10.1016/S0006-2952(03)00114-X
Funding
This work was supported by Brazilian National Council of Technological and Scientific Development (CNPq), “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES), “Programa de Apoio a Núcleos Emergentes” (PRONEM) MCTI/CNPq [Grant number 472669/2011-7, 475896/2012-2], and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CAPES/PROEX [process number: 23038.005848/2018-31]. FAAS received a fellowship from CNPq. PCR, DDH, STS, JLC, MRL and NRC received a fellowship from CAPES.
Author information
Authors and Affiliations
Contributions
All authors were involved in the development of this manuscript. As the corresponding author, Rômulo P. Barcelos oversaw the complete manuscript development. The study was designed by José L. Cechella; the training was designed and performed by Marlon R. Leite; data were collected and analyzed by Martin T. B. Leite, Micaela B. Souza, Thayanara C. da Silva and Nelson R. De Carvalho; data interpretation and article preparation were undertaken by Pamela C. Da Rosa, Diane D. Hartmann, and Sílvio T. Stefanello; the study conceived and supervisioned, and review of final version by Félix A. A. Soares, Gustavo O. Puntel. All authors have approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The author declares that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Da Rosa, P.C., Hartmann, D.D., Stefanello, S.T. et al. Diphenyl diselenide blunts swimming training on mitochondrial liver redox adaptation mechanisms of aged animals. Sport Sci Health 16, 281–290 (2020). https://doi.org/10.1007/s11332-019-00603-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-019-00603-8