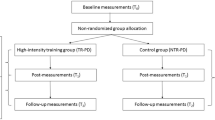
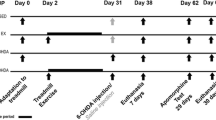
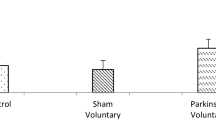
Abstract
Parkinson’s disease (PD) is a neurodegenerative disease characterized by a progressive degeneration of dopaminergic neurons in the substantia nigra pars compact (SNpc). Previous studies have shown that cognitive deficits and motor impairment symptoms seen in PD and that physical exercise may exert beneficial effects on PD. In most cases, brain-derived neurotrophic factor (BDNF) is involved in such effects. However, little is known on the role of BDNF in exercise, especially high-intensity interval training (HIIT)-related effects on PD. The aim of this study was to investigate the effects of 6 weeks HIIT against experimentally reserpine (RES)-induced PD in male mice, by analyzing the motor coordination, learning and serum BDNF level. Male mice received 20 (s.c) injections of 0.1 mg/kg RES or vehicle, every other day. Rotarod and spontaneous alternation tests were used for measurement of motor coordination and short-term memory, respectively, and serum levels of BDNF were also measured using the ELISA technique. All behavioral tests were performed 48 h after the RES injection. RES injection caused a significant motor coordination and cognitive deficits (p < 0.05) and these effects were reversed in mice after receiving exercise protocol. HIIT improved the motor coordination and cognitive performance against RES administration (p < 0.05). Also, serum BDNF level was decreased in mice RES-induced PD (p < 0.05) and HIIT restored this to control levels (p < 0.05). Taken together, our results suggest that HIIT shows a protective effect in a mice model of PD and may repair motor coordination and cognitive dysfunctions in PD due to increased serum levels of BDNF.



Similar content being viewed by others
References
de Lau LML, Breteler MMB (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535. https://doi.org/10.1016/s1474-4422(06)70471-9
Pringsheim T, Jette N, Frolkis A, Steeves TDL (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Dis 29(13):1583–1590. https://doi.org/10.1002/mds.25945
Wu T, Wang J, Wang C, Hallett M, Zang Y, Wu X et al (2012) Basal ganglia circuits changes in Parkinson’s disease patients. Neurosci Lett 524(1):55–59. https://doi.org/10.1016/j.neulet.2012.07.012
Miller DB, O’Callaghan JP (2015) Biomarkers of Parkinson’s disease: present and future. Metabolism 64(3):S40–S46. https://doi.org/10.1016/j.metabol.2014.10.030
Peres FF, Levin R, Suiama MA, Diana MC, Gouvêa DA, Almeida V et al (2016) Cannabidiol prevents motor and cognitive impairments induced by reserpine in rats. Front Pharmacol. https://doi.org/10.3389/fphar.2016.00343
Campêlo CLC, Santos JR, Silva AF, Dierschnabel AL, Pontes A, Cavalcante JS et al (2017) Exposure to an enriched environment facilitates motor recovery and prevents short-term memory impairment and reduction of striatal BDNF in a progressive pharmacological model of parkinsonism in mice. Behav Brain Res 328:138–148. https://doi.org/10.1016/j.bbr.2017.04.028
Lins LCRF, Souza MF, Bispo JMM, Gois AM, Melo TCS, Andrade RAS et al (2018) Carvacrol prevents impairments in motor and neurochemical parameters in a model of progressive parkinsonism induced by reserpine. Brain Res Bull 139:9–15. https://doi.org/10.1016/j.brainresbull.2018.01.017
Aguiar AS, Araújo AL, Da-Cunha TR, Speck AE, Ignácio ZM, De-Mello V et al (2009) Physical exercise improves motor and short-term social memory deficits in reserpinized rats. Brain Res Bull 79(6):452–457. https://doi.org/10.1016/j.brainresbull.2009.05.005
Fernandes VS, Ribeiro AM, Melo TG, Godinho M, Barbosa FF, Medeiros DS et al (2008) Memory impairment induced by low doses of reserpine in rats: Possible relationship with emotional processing deficits in Parkinson disease. Progr Neuro-Psychopharmacol Biol Psychiatry 32(6):1479–1483. https://doi.org/10.1016/j.pnpbp.2008.05.004
Santos JR, Cunha JAS, Dierschnabel AL, Campêlo CLC, Leão AHFF, Silva AF et al (2013) Cognitive, motor and tyrosine hydroxylase temporal impairment in a model of parkinsonism induced by reserpine. Behav Brain Res 253:68–77. https://doi.org/10.1016/j.bbr.2013.06.031
Sasi M, Vignoli B, Canossa M, Blum R (2017) Neurobiology of local and intercellular BDNF signaling. Pflügers Archiv Eur J Physiol 469(5-6):593–610. https://doi.org/10.1007/s00424-017-1964-4
Van der Kolk NM, Speelman AD, van Nimwegen M, Kessels RPC, IntHout J, Hakobjan M et al (2015) BDNF polymorphism associates with decline in set shifting in Parkinson’s disease. Neurobiol Aging 36(3):1605.e1–1605.e6. https://doi.org/10.1016/j.neurobiolaging.2014.08.02
Scalzo P, Kümmer A, Bretas TL, Cardoso F, Teixeira AL (2009) Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J Neurol 257(4):540–545. https://doi.org/10.1007/s00415-009-5357-2
Lee J, Shin ME, Ji E, Kim TW, Cho H, Kim CJ et al (2014) Treadmill exercise improves motor coordination through ameliorating Purkinje cell loss in amyloid beta23-35-induced Alzheimer’s disease rats. J Exerc Rehabil 10(5):258–264. https://doi.org/10.12965/jer.140163
Lee JM, Kim TW, Park SS, Han JH, Shin MS, Lim BV et al (2018) Treadmill exercise improves motor function by suppressing purkinje cell loss in parkinson disease rats. Int Neurourol J 22(Suppl 3):S147–S155. https://doi.org/10.5213/inj.1836226.113
Chou W, Liu YF, Lin CH, Lin MT, Chen CC, Liu WP et al (2018) Exercise rehabilitation attenuates cognitive deficits in rats with traumatic brain injury by stimulating the cerebral HSP20/BDNF/TrkB signalling axis. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1011-2
Chen YH, Kuo TT, Kao JH, Huang EYK, Hsieh TH, Chou YC et al (2018) Exercise ameliorates motor deficits and improves dopaminergic functions in the rat hemi-Parkinson’s model. Sci Rep. https://doi.org/10.1038/s41598-018-22462-y
Picelli A, Varalta V, Melotti C, Zatezalo V, Fonte C, Amato S et al (2016) Effects of treadmill training on cognitive and motor features of patients with mild to moderate Parkinson’s disease: a pilot, single-blind, randomized controlled trial. Funct Neurol 31(1):25–31. doi: https://doi.org/10.11138/fneur/2016.31.1.025
de Oliveira RT, Felippe LA, Bucken Gobbi LT, Barbieri FA, Christofoletti G (2017) Benefits of exercise on the executive functions in people with Parkinson disease: a controlled clinical trial. Am J Phys Med Rehabil 96(5):301–306. https://doi.org/10.1097/phm.0000000000000612
Zoladz JA, Majerczak J, Zeligowska E, Mencel J, Jaskolski A, Jaskolska A et al (2014) Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J Physiol Pharmacol 65(3):441–448
Jiménez-Maldonado A, Rentería I, García-Suárez PC, Moncada-Jiménez J, Freire-Royes LF (2018) The impact of high-intensity interval training on brain derived neurotrophic factor in brain: a mini-review. Front Neurosci. https://doi.org/10.3389/fnins.2018.00839
Marusiak J, Żeligowska E, Mencel J, Kisiel-Sajewicz K, Majerczak J, Zoladz J et al (2015) Interval training-induced alleviation of rigidity and hypertonia in patients with Parkinson’s disease is accompanied by increased basal serum brain-derived neurotrophic factor. J Rehabil Med 47(4):372–375. https://doi.org/10.2340/16501977-1931
Songstad NT, Kaspersen KHF, Hafstad AD, Basnet P, Ytrehus K, Acharya G (2015) Effects of high intensity interval training on pregnant rats, and the placenta, heart and liver of their fetuses. PLoS One 10(11):e0143095. https://doi.org/10.1371/journal.pone.0143095
Martinez-Huenchullan SF, Maharjan BR, Williams PF, Tam CS, Mclennan SV, Twigg SM (2018) Differential metabolic effects of constant moderate versus high intensity interval training in high-fat fed mice: possible role of muscle adiponectin. Physiol Rep 6(4):e13599. https://doi.org/10.14814/phy2.13599
Bak J, Pyeon HI, Seok JI, Choi YS (2017) Effect of rotation preference on spontaneous alternation behavior on Y maze and introduction of a new analytical method, entropy of spontaneous alternation. Behav Brain Res 320:219–224. https://doi.org/10.1016/j.bbr.2016.12.011
Mätlik K, Võikar V, Vilenius C, Kulesskaya N, Andressoo J (2018) Two-fold elevation of endogenous GDNF levels in mice improves motor coordination without causing side-effects. Sci Rep 8:11861. https://doi.org/10.1038/s41598-018-29988-1
de Almeida AA, Gomes da Silva S, Lopim GM, Vannucci Campos D, Fernandes J, Cabral FR (2017) Resistance exercise reduces seizure occurrence, attenuates memory deficits and restores bdnf signaling in rats with chronic epilepsy. Neurochem Res 42(4):1230–1239. https://doi.org/10.1007/s11064-016-2165-9
Li J, Zhang S, Li C, Li M, Ma L (2018) Sitagliptin rescues memory deficits in Parkinsonian rats via upregulating BDNF to prevent neuron and dendritic spine loss. Neurol Res 40(9):736–743. https://doi.org/10.1080/01616412.2018.1474840
Park BK, Kim YR, Kim YH, Yang C, Seo CS, Jung IC et al (2018) Antidepressant-like effects of Gyejibokryeong-hwan in a mouse model of reserpine-induced depression. BioMed Res Int 2018:1–12. https://doi.org/10.1155/2018/5845491
Zuccato C, Marullo M, Vitali B, Tarditi A, Mariotti C, Valenza M et al (2011) Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS One 6(8):e22966. https://doi.org/10.1371/journal.pone.0022966
Autry AE, Monteggia LM (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64(2):238–258. https://doi.org/10.1124/pr.111.005108
Soares AT, Andreazza AC, Rej S, Rajji TK, Gildengers AG, Lafer B et al (2016) Decreased brain-derived neurotrophic factor in older adults with bipolar disorder. Am J Geriatr Psychiatry 24(8):596–601. https://doi.org/10.1016/j.jagp.2016.02.052
Lieberwirth C, Pan Y, Liu Y, Zhang Z, Wang Z (2016) Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory. Brain Res 1644:127–140. https://doi.org/10.1016/j.brainres.2016.05.015
Alam MJ, Kitamura T, Saitoh Y, Ohkawa N, Kondo T, Inokuchi K (2018) Adult neurogenesis conserves hippocampal memory capacity. J Neurosci 38(31):6854–6863. https://doi.org/10.1523/jneurosci.2976-17.2018
Zimmermann T, Remmers F, Lutz B, Leschik J (2016) ESC-derived BDNF-overexpressing neural progenitors differentially promote recovery in Huntington’s disease models by enhanced striatal differentiation. Stem cell Rep 7(4):693–706. https://doi.org/10.1016/j.stemcr.2016.08.018
Mellesmoen A, Sheeler C, Ferro A, Rainwater O, Cvetanovic M (2019) Brain derived neurotrophic factor (BDNF) delays onset of pathogenesis in transgenic mouse model of spinocerebellar ataxia type 1 (SCA1). Front Cell Neurosci 12:509. https://doi.org/10.3389/fncel.2018.00509
Nguyen KQ, Rymar VV, Sadikot AF (2016) Impaired TrkB signaling underlies reduced BDNF-mediated trophic support of striatal neurons in the R6/2 mouse model of Huntington’s disease. Front Cell Neurosci 10:37. https://doi.org/10.3389/fncel.2016.00037
Baydyuk M, Xu B (2014) BDNF signaling and survival of striatal neurons. Front Cell Neurosci 8:254. https://doi.org/10.3389/fncel.2014.00254
Fleitas C, Piñol-Ripoll G, Marfull P, Rocandio D, Ferrer I, Rampon C et al (2018) proBDNF is modified by advanced glycation end products in Alzheimer’s disease and causes neuronal apoptosis by inducing p75 neurotrophin receptor processing. Mol Brain. https://doi.org/10.1186/s13041-018-0411-6
Zhao X, Chen XQ, Han E, Hu Y, Paik P, Ding Z et al (2016) TRiC subunits enhance BDNF axonal transport and rescue striatal atrophy in Huntington’s disease. Proc Natl Acad Sci 113(38):E5655–E5664. https://doi.org/10.1073/pnas.1603020113
Ma Q, Yang J, Li T, Milner TA, Hempstead BL (2015) Selective reduction of striatal mature BDNF without induction of proBDNF in the zQ175 mouse model of Huntington’s disease. Neurobiol Dis 82:466–477. https://doi.org/10.1016/j.nbd.2015.08.008
Guo Z, Rudow G, Pletnikova O, Codispoti KE, Orr BA, Crain BJ et al (2012) Striatal neuronal loss correlates with clinical motor impairment in Huntington’s disease. Mov Disord 27(11):1379–1386. https://doi.org/10.1002/mds.25159
Baydyuk M, Xie Y, Tessarollo L, Xu B (2013) Midbrain-derived neurotrophins support survival of immature striatal projection neurons. J Neurosci 33(8):3363–3369. https://doi.org/10.1523/jneurosci.3687-12.2013
Hernandez-Chan NG, Bannon MJ, Orozco-Barrios CE, Escobedo L, Zamudio S, De la Cruz F et al (2015) Neurotensin-polyplex-mediated brain-derived neurotrophic factor gene delivery into nigral dopamine neurons prevents nigrostriatal degeneration in a rat model of early Parkinson’s disease. J Biomed Sci. https://doi.org/10.1186/s12929-015-0166-7
Liu PZ, Nusslock R (2018) Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. https://doi.org/10.3389/fnins.2018.00052
Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T et al (2010) Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res 1310:200–207. https://doi.org/10.1016/j.brainres.2009.10.075
Afzalpour ME, Chadorneshin HT, Foadoddini M, Eivari HA (2015) Comparing interval and continuous exercise training regimens on neurotrophic factors in rat brain. Physiol Behav 147:78–83. https://doi.org/10.1016/j.physbeh.2015.04.012
Freitas DA, Rocha-Vieira E, Soares BA, Nonato LF, Fonseca SR, Martins JB et al (2018) High intensity interval training modulates hippocampal oxidative stress, BDNF and inflammatory mediators in rats. Physiol Behav 184:6–11. https://doi.org/10.1016/j.physbeh.2017.10.027
Bakowiec-Iskra E, Vermehren-Schmaedick A, Balkowiec A (2011) Tumor necrosis factor-alpha increases brain-derived neurotrophic factor expression in trigeminal ganglion neurons in an activity-dependent manner. Neuroscience 180:322–333. https://doi.org/10.1016/j.neuroscience.2011.02.028
Pugazhenthi S, Nesterova A, Jambal P, Audesirk G, Kern M, Cabell L et al (2003) Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J Neurochem 84:982–996. https://doi.org/10.1046/j.1471-4159.2003.01606.x
Fisher G, Brown AW, Bohan Brown MM, Alcorn A, Noles C, Winwood L et al (2015) High intensity interval- vs moderate intensity- training for improving cardiometabolic health in overweight or obese males: a randomized controlled trial. PLoS One 10:e0138853. https://doi.org/10.1371/journal.pone.0138853
Daabis R, Hassan M, Zidan M (2017) Endurance and strength training in pulmonary rehabilitation for COPD patients. Egypt J Chest Dis Tuberc 66:231–236. https://doi.org/10.1016/j.ejcdt.2016.07.003
Weston KS, Wisløff U, Coombes JS (2014) High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48:1227–1234. https://doi.org/10.1136/bjsports-2013-092576
Gibala MJ, Jones AM (2013) Physiological and performance adaptations to high-intensity interval training. Nestle Nutr Inst Workshop Ser 76:51–60. https://doi.org/10.1159/000350256
Gillen JB, Gibala MJ (2013) Is high-intensity interval training a time efficient exercise strategy to improve health and fitness? Appl Physiol Nutr Metab 39:409–412. https://doi.org/10.1139/apnm-2013-0187
Saanijoki T, Nummenmaa L, Koivumäki M, Löyttyniemi E, Kalliokoski KK, Hannukainen JC (2018) Affective adaptation to repeated SIT and MICT protocols in insulin-resistant subjects. Med Sci Sports Exerc 50:18–27. https://doi.org/10.1249/mss.0000000000001415
Heisz JJ, Tejada MG, Paolucci EM, Muir C (2016) Enjoyment for high-intensity interval exercise increases during the first six weeks of training: implications for promoting exercise adherence in sedentary adults. PLoS One 11:e0168534. https://doi.org/10.1371/journal.pone.0168534
Marquez CM, Vanaudenaerde B, Troosters T, Wenderoth N (2015) High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J Appl Physiol 119(12):1363–1373. https://doi.org/10.1152/japplphysiol.00126.2015
Chapman JJ, Coombes JS, Brown WJ, Khan A, Chamoli S, Pachana NA et al (2017) The feasibility and acceptability of high-intensity interval training for adults with mental illness: a Pilot Study. Mental Health Phys Act 13:40–48. https://doi.org/10.1186/s40814-017-0133-z
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All procedures performed in this study involving animal participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sabaghi, A., Heirani, A., Mahmoodi, H. et al. High-intensity interval training prevents cognitive-motor impairment and serum BDNF level reduction in parkinson mice model. Sport Sci Health 15, 681–687 (2019). https://doi.org/10.1007/s11332-019-00586-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-019-00586-6