Abstract
Objective
By observing the differences in sleep parameters between portable sleep monitoring (PM) and polysomnography (PSG) in children, we aimed to investigate the diagnostic value and feasibility of PM in children with suspected obstructive sleep apnea (OSA).
Study design
This prospective study enrolled consecutive children (aged 3–14 years) with suspected OSA in Shenzhen Children’s Hospital. They had PSG and PM in the sleep laboratory. Clinical parameters of the two sleep monitoring methods were compared.
Results
A total of 58 children participated. They were classified into two groups according to age: 28 children aged 3 to 5 years and 30 children aged 6 to 14 years. No significant differences were observed in apnea-hypopnea index (AHI), lowest oxygen saturation (LSaO2), and mean oxygen saturation (MSaO2) between PM and PSG, but the sleep efficiency with PM was significantly higher (3–5 years age: 92.2 ± 11.3% vs 85.2 ± 14.3%, 6–14 years age: 93.2 ± 14.5% vs 84.8 ± 16.3%, both P < 0.05) than the sleep efficiency with PSG. Pearson correlation analysis indicated a strong correlation between AHI, LSaO2, MSaO2, and sleep efficiency measured by PSG and PM. Receiver operating characteristic curve (ROC) analysis showed that PM was a reliable diagnostic tool for OSA. PM has high sensitivity (3–5 years age: 95.8%, 6–14 years age: 96.3%) and low specificity (3–5 years age: 25.0%, 6–14 years age: 33.3%) for OSA in children. Thus, there is a low rate of missed diagnoses, but there is some inaccuracy in excluding children who do not have OSA.
Conclusion
The results showed that PM has a good correlation with the various parameters of PSG. PM may be a reliable tool for diagnosing moderate and severe OSA in children, especially those who cannot cooperate with PSG or who have limited access to PSG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common disease in children, with a prevalence of 1.2% to 5.7% [1]. Its symptoms include snoring, mouth breathing, apnea, frequent awakenings, bed-wetting, sweating, and hyperactivity. Occasionally, excessive daytime sleepiness may occur, which can seriously affect the child’s health and development, including growth retardation, cognitive and behavioral abnormalities, cardiovascular changes, and pulmonary hypertension [1, 2]. Enlargement of the adenoids and tonsils is the main cause of OSA in children, but persistent sleep-disordered breathing occurs in 33.7% of children after adenotonsillectomy [3, 4]. Therefore, accurate diagnosis and clear etiology are important to guide appropriate treatments.
Polysomnography (PSG) is the “gold standard” for diagnosing OSA and is the most commonly used method for diagnosing OSA. However, PSG has limitations, such as the need for expensive equipment and a sleep monitoring room, the need for professional staff, and the influence of multiple electrodes on the patient’s sleep. Some pediatric patients cannot cooperate with PSG, and patients must often wait a long time for a study, limiting its usefulness. In the USA, only about 10% of children undergo sleep apnea monitoring before undergoing adenoid and tonsil surgery, which means that many children have undergone surgical risks without an accurate diagnosis of OSA [5]. Practically, the diagnosis of OSA in children mostly relies on medical history and physical examination, while complaints of guardians and physical examinations such as adenoid tonsil size have no correlation with the diagnosis and severity of OSA in children [6,7,8]. Therefore, there is a need for a more economical and convenient diagnostic tool.
According to standards set by the American Academy of Sleep Medicine, sleep-related breathing tests are classified into four levels [9]. Portable sleep monitoring devices refer to types II-IV devices [9]. There are various types of sleep monitoring devices used clinically. In addition to the “gold standard” type I PSG, there are also type II portable monitoring (PM) devices, type III PM devices (such as the Stardust II, SOMNO medics), and Type IV PM devices (such as the Watch PAT system, micro-motion sensor sleep system, Morpheus Ox system, actigraphy, and pulse oximeter). Devices used for PM are small and lightweight. PM is a method of diagnosing sleep disorders by simplifying the biological electrodes. With the increasingly mature technology of PM devices, PM is gradually becoming more reliable for diagnosing OSA. In adults, studies have demonstrated agreement between a PSG and type III PMs for diagnosing OSA [10, 11]. However, such PM validation studies are scarce in pediatric patients. Validation studies based on adults cannot be extrapolated to children as the pathophysiology and management in children differ from those in adults [12]. This study aimed to analyze the parameters of PM and PSG in the same individuals to determine the value of PM in diagnosing OSA in children.
Patients and methods
Patients
This study included children who were admitted to the Otolaryngology Department of Shenzhen Children’s Hospital between March 2017 and January 2018. Consecutive children aged 3–14 years undergoing clinically indicated PSG to evaluate possible OSA were invited to participate with permission of parents or guardians. Children with obesity, Down syndrome, craniofacial malformations, sickle cell disease, neuromuscular disease, and mucopolysaccharide diseases were excluded. This prospective study was approved by the Ethics Committee of Shenzhen Childrens Hospital.
Study design
According to a random number table, the subjects were assigned to have PM first or PSG first. They would complete PSG and PM on separate nights, the two tests occurring within 3 days. PSG and PM were conducted at Shenzhen Children’s Hospital Sleep Laboratory and were performed by sleep technicians with considerable experience in performing pediatric sleep studies.
PSG (SOMNO medics, V5) monitoring parameters included electroencephalogram (EEG) (8-lead), electronystagmogram, electrocardiogram (ECG), mouth and nasal airflow (thermistor and pressure monitoring), respiratory movement (thoracic and abdominal), finger blood oxygen saturation, postures, and mandible myoelectricity. The monitoring parameters of PM (SOMNO medics, Germany) included nasal airflow (pressure monitoring), finger blood oxygen saturation, and respiratory movement (thoracic and abdominal). Monitoring was conducted from 21:30 to 06:30 the next day. The original data were automatically recorded and analyzed, and then manually corrected.
All sleep scoring followed the guidelines of the AASM manual [9]. Apnea was defined as the cessation of airflow in the mouth and nose during sleep with thoracic and abdominal respiration. Hypopnea was defined as a ≥ 30% decrease in airflow signal amplitude lasting ≥ 10 s and accompanied by ≥ 3% oxygen desaturation. The time duration of respiratory events was defined as two or more respiratory cycles. PSG and PM parameters examined included the total sleep apnea–hypopnea index (AHI), mean oxygen saturation (MSaO2), lowest pulse oxygen saturation (LSaO2), and sleep efficiency. The diagnosis of OSA was based on the AHI, with categories of mild, moderate, and severe OSA defined by an AHI of 1 to 5, 6 to 10, and > 10 events/h, respectively [13].
Statistical analysis
The statistical analysis in this study was conducted using SPSS version 26.0. All tests were two-tailed, with a significance level set at α = 0.05. A P-value less than 0.05 was considered statistically significant. The measurement data were presented as mean ± SD. AHI, LSaO2, MSaO2, and sleep efficiency compared by group using t test after testing for normal distribution. Correlation analysis was assessed using the Pearson correlation analysis. PSG results were used as the gold standard for diagnosing OSA. ROC curve analysis was performed to measure the area under the ROC curve (AUC). To further evaluate classification prediction, cross tabulation was used to calculate sensitivity and specificity.
Results
General data
A total of 58 children were enrolled. They were classified into two groups according to age: group 1 (3 to 5 years, 28 cases) and group 2 (6 to 14 years, 30 cases). Thirty-three children were males and 25 were females. Their average age was 7.3 ± 3.3 years. According to the reference for Chinese children and adolescents [8], no subject was overweight or obese.
Comparison of diagnosis between PSG and PM
The manually scored PSG results were used as the gold standard. The comparison of diagnosis between PSG and PM is summarized in Table 1. In the 3–5 years age group, PSG diagnosed primary snoring (PS) in 3 children who were diagnosed with mild OSA by PM 1 child diagnosed with mild OSA by PSG was classified as PS by PM, 1 child diagnosed with mild OSA by PSG was classified as moderate OSA by PM, and 1 child diagnosed with moderate OSA by PSG was classified as severe OSA by PM, while diagnosis results were consistent for other children. In the 6–14 years age group, PSG diagnosed PS in 2 children who were diagnosed with mild OSA by PM, 1 child diagnosed with mild OSA by PSG was classified as PS by PM, and 1 child diagnosed with severe OSA by PSG was classified as moderate OSA by PM, and diagnosis results for the remaining children by the two methods were consistent.
Correlation between PSG parameters and PM parameters
There were no statistically significant differences in AHI, LSaO2, and MSaO2 between the two age groups (3–5 years and 6–14 years, P > 0.05), and the PM sleep efficiency was significantly higher (P < 0.05) than the PSG sleep efficiency, as indicated in Table 2. When comparing the AHI values between the two groups, the PM values were slightly higher than the PSG values, but this difference was not statistically significant (P > 0.05).
Further Pearson correlation analysis was conducted on the AHI, LSaO2, MSaO2, and sleep efficiency of the two groups of children monitored by the two devices, and statistically significant differences were observed (P < 0.05). Both PM and PSG monitoring showed good correlations for various sleep parameters in both the 3–5 years and 6–14 years age groups.
Sensitivity and specificity of PM
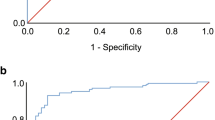
Using AHI-PSG ≥ 1 times/h as the diagnostic threshold for OSA in children, ROC analysis was performed to compare the diagnostic consistency of PM between the simple snoring group and the OSA group. The AUC for children aged 3–5 years was 0.97 (95% CI: 0.90–1.00, P = 0.003), with the sensitivity and specificity both at 95.8% and 25.0%, respectively, as shown in Fig. 1. Similarly, the AUC for children aged 6–14 years was 0.96 (95% CI: 0.89–1.00, P = 0.009), with the sensitivity and specificity both at 96.3% and 33.3%, respectively, as shown in Fig. 2. Results pertaining to classification prediction; ROC curve analyses and cross tabulation, are shown in Tables 3 and 4. These findings indicate that PM is a reliable predictor of OSA in children.
Discussion
The PM used in this study (SOMNO medics, Germany) measures nasal airflow (using a pressure sensor), finger pulse oximetry, and respiratory movement. The findings demonstrated a close agreement between PSG and PM in children. No significant differences were observed in AHI, LSaO2, and MSaO2 between PM and PSG, but the PM sleep efficiency was significantly higher than the PSG sleep efficiency. ROC analysis showed that PM was a reliable diagnostic tool for OSA, with PM having high sensitivity (3–5 years age: 95.8%, 6–14 years age: 96.3%) but low specificity (3–5 years age: 25.0%, 6–14 years age: 33.3%) for OSA in children. Thus, there was a low rate of missed diagnoses, but there was inaccuracy in excluding children without OSA.
In recent years, studies have reported on the correlation of various monitoring parameters between PM and PSG and found no significant differences in the main parameters, suggesting that PM has a high sensitivity, specificity, and accuracy for the diagnosis of OSA in adults [14,15,16]. In fact, portable sleep monitoring has become a fundamental method for assessing sleep-disordered breathing in adults [16, 17]. However, the pathophysiology and management of OSA in children are not the same as that in adults and differs among various age groups. The sensitivity and specificity of the PM in diagnosing OSA in children has had large fluctuations due in part to the type of the PM used in different studies [18]. Tan et al. [19] used home sleep monitoring (respiratory polygraphy, RP) in 100 pediatric patients with suspected OSA and applied it with simultaneous PSG. Their findings, when using PSG-AHI ≥ 5 times/h, revealed a sensitivity of 62.5%, specificity of 100%, positive predictive value of 100%, negative predictive value of 80%, and an AUC of 0.81, and these results indicated a strong correlation between the AHI values derived from both methods. Weimin et al. [20] observed a significant positive correlation between WatchPAT-AHI and PSG-AHI (Pearson’s correlation coefficient, r = 0.92, P < 0.001) [18]. In a comparative study by Cheung et al. [21] involving 45 pediatric patients suspected OSA, RP demonstrated a strong correlation with PSG-derived AHI (r = 0.98, P < 0.001). Masoud et al. [22] used MediByte in 70 pediatric participants (median age 10.8 years) and applied it with simultaneous PSG. They found that for an AHI cutoff of ≥ 5 times/h, the AUC was 0.89, the sensitivity was 95.5%, and the specificity was 66.7%; for an AHI cutoff of ≥ 10 times/h, the AUC was 1.00, the sensitivity was 100.0%, and the specificity was 93.4%. Lesser et al. [23], in a study involving 25 obese children with an average age of 13.6 years, utilized Apnealink in conjunction with PSG monitoring. They found that for an AHI cutoff of ≥ 5 times/h, Apnealink exhibited a sensitivity of 85.7% and specificity of 83.3%.
AHI is the main index for assessing the severity of OSA. The literature shows conflicting results with some studies showing PM overestimating AHI [12, 23], whereas others show PM underestimating AHI [24, 25]. However, in this study, the PM-AHI value was generally higher than the PSG-AHI value. Due to the lack of EEG monitoring with PM, changes in the sleep structure cannot be observed, and fluctuations in the respiratory rate during sleep may be mistakenly identified as wakefulness, resulting in a reduced calculation of sleep efficiency and an increase in the number of apnea or hypopnea events per hour. At the same time, false positives may be generated from limb movements during sleep. Underestimation of the AHI may also occur due to the number of apneas being divided by total recorded time rather than total sleep time [24, 25]. Therefore, the use of a nighttime sleep log may assist in the interpretation of sleep–wake periods measured by PM. This will likely increase the validity and reproducibility of portable devices.
Each diagnostic modality clearly has its own set of advantages and disadvantages. PSG requires the establishment of a sleep laboratory with professional personnel on night duty with inherent costs. Due to time constraints, limited funds, equipment, and personnel, PSG has not met the clinical demands. Furthermore, for children, PSG with leads and electrode stickers causes disruption of sleep, and poor cooperation from pediatric patients makes large-scale screening impossible. However, PSG may also provide additional valuable information. The inclusion of EEG data in PSG recordings enables the assessment of various aspects of sleep and neurological activity. This data can help in characterizing different sleep stages, detecting abnormal brain wave patterns, and diagnosing sleep disorders such as sleep apnea, insomnia, and narcolepsy. Therefore, PSG may be a good choice for patients with significant comorbid medical conditions that may degrade the accuracy of PM and patients suspected of having comorbid sleep disorders. PM is a convenient, low-cost, and easy-to-use tool with high sensitivity for diagnosing OSA in children, but there is some inaccuracy in identifying children who do not have OSA. It is also important to consider other factors regarding the diagnosis of OSA, such as symptoms, other test results, and risk factors, when interpreting results of sleep tests [18].
This study has several evident limitations. First, the small sample size undermines the representativeness of the findings. The participants consisted solely of children referred to a sleep clinic for investigation of OSA who were otherwise healthy. Second, the study exclusively assessed the use of PM in an inpatient setting without validating its applicability in a home environment. Third, the study relied on single-night sleep monitoring rather than continuous monitoring over multiple nights. This approach may potentially result in missing positive cases or misclassifying disease groups due to confounding factors such as the first-night effect. Fourth, the PM employed in this study lacked the capability to differentiate EEG data and perform sleep staging. Consequently, it may lead to underdiagnosis of other sleep disorder-related illnesses.
In summary, the PM accurately identifies OSA when tested in a sleep laboratory on pediatric patients with suspected OSA referred for symptoms of sleep-related breathing disorder. The PM-AHI strongly correlated with the PSG-AHI. And the sensitivity for detection of moderate and severe OSAHS diagnosed by PSG using PM was very high. PM may play an important role in diagnosing moderate and severe OSA, especially in patients who cannot cooperate with PSG or who have limited access to PSG.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J et al (2012) Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 130:576–584
Franco RA Jr, Rosenfeld RM, Rao M (2000) First place–resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg: official journal of American Academy of Otolaryngology-Head and Neck Surgery 123:9–16
Friedman M, Wilson M, Lin HC, Chang HW (2009) Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg : official journal of American Academy of Otolaryngology-Head and Neck Surgery 140:800–8
Isaiah A, Hamdan H, Johnson RF, Naqvi K, Mitchell RB (2017) Very severe obstructive sleep apnea in children: outcomes of adenotonsillectomy and risk factors for persistence. Otolaryngol Head Neck Surg : official journal of American Academy of Otolaryngology-Head and Neck Surgery 157:128–34
Roland PS, Rosenfeld RM, Brooks LJ, Friedman NR, Jones J, Kim TW et al (2011) Clinical practice guideline: polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg : official journal of American Academy of Otolaryngology-Head and Neck Surgery 145:S1-15
Brooks LJ, Stephens BM, Bacevice AM (1998) Adenoid size is related to severity but not the number of episodes of obstructive apnea in children. J Pediatr 132:682–686
Wu Y, Zheng L, Cui G, Xu Z, Ni X (2022) Subtypes of obstructive sleep apnea in children and related factors. J Clin Sleep Med : JCSM : official publication of the American Academy of Sleep Medicine 18:2397–2404
Xiao L, Su S, Liang J, Jiang Y, Shu Y, Ding L (2022) Analysis of the risk factors associated with obstructive sleep apnea syndrome in Chinese children. Front Pediatr 10:900216
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK et al (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med : JCSM : official publication of the American Academy of Sleep Medicine 8:597–619
Xu L, Han F, Keenan BT, Kneeland-Szanto E, Yan H, Dong X et al (2017) Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in Chinese adults. J Clin Sleep Med : JCSM : official publication of the American Academy of Sleep Medicine 13:675–683
Cooksey JA, Balachandran JS (2016) Portable monitoring for the diagnosis of OSA. Chest 149:1074–1081
Massicotte C, Al-Saleh S, Witmans M, Narang I (2014) The utility of a portable sleep monitor to diagnose sleep-disordered breathing in a pediatric population. Can Respir J 21:31–35
(2014) Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 120:268–86
Yin M, Miyazaki S, Ishikawa K (2006) Evaluation of type 3 portable monitoring in unattended home setting for suspected sleep apnea: factors that may affect its accuracy. Otolaryngol Head Neck Surg : official journal of American Academy of Otolaryngology-Head and Neck Surgery 134:204–9
To KW, Chan WC, Chan TO, Tung A, Ngai J, Ng S et al (2009) Validation study of a portable monitoring device for identifying OSA in a symptomatic patient population. Respirology (Carlton, Vic) 14:270–275
Blackman A, McGregor C, Dales R, Driver HS, Dumov I, Fleming J et al (2010) Canadian Sleep Society/Canadian Thoracic Society position paper on the use of portable monitoring for the diagnosis of obstructive sleep apnea/hypopnea in adults. Can Respir J 17:229–232
Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K et al (2017) Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med : JCSM : official publication of the American Academy of Sleep Medicine 13:479–504
Tuohuti A, Lin Z, Cai J, Chen X (2023) Can portable sleep monitors replace polysomnography for diagnosis of pediatric OSA: a systematic review and meta-analysis. Euro Arch Oto-Rhino-Laryngol: official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery 280(10):4351–4359
Tan HL, Gozal D, Ramirez HM, Bandla HP, Kheirandish-Gozal L (2014) Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. Sleep 37:255–260
Weimin L, Rongguang W, Dongyan H, Xiaoli L, Wei J, Shiming Y (2013) Assessment of a portable monitoring device WatchPAT 200 in the diagnosis of obstructive sleep apnea. Euro Arch Oto-rhino-laryngol : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery 270:3099–105
Cheung TW, Lam DS, Chan PC, Yau PS, Yeung KW (2021) Comparing respiratory polygraphy with pulse transit time analysis versus overnight polysomnography in the diagnosis of obstructive sleep apnoea in children. Sleep Med 81:457–462
Masoud AI, Patwari PP, Adavadkar PA, Arantes H, Park C, Carley DW (2019) Validation of the MediByte portable monitor for the diagnosis of sleep apnea in pediatric patients. J Clin Sleep Med : JCSM : official publication of the American Academy of Sleep Medicine 15:733–742
Lesser DJ, Haddad GG, Bush RA, Pian MS (2012) The utility of a portable recording device for screening of obstructive sleep apnea in obese adolescents. J Clin Sleep Med : JCSM : official publication of the American Academy of Sleep Medicine 8:271–277
Tan HL, Kheirandish-Gozal L, Gozal D (2015) Pediatric home sleep apnea testing: slowly getting there! Chest 148:1382–1395
Pereira EJ, Driver HS, Stewart SC, Fitzpatrick MF (2013) Comparing a combination of validated questionnaires and level III portable monitor with polysomnography to diagnose and exclude sleep apnea. J Clin Sleep Med : JCSM : official publication of the American Academy of Sleep Medicine 9:1259–1266
Acknowledgements
The authors are extremely grateful for the enthusiasm and time provided by the parents and participants in this study.
Funding
This work was supported by Guangdong High-level Hospital Construction Fund Clinical Research Project of Shenzhen Children’s Hospital (no. LCYJ2022063).
Author information
Authors and Affiliations
Contributions
ZX.X, X.W, YC.C, and YS.T contributed to the study conception and design; data acquisition, analysis, and interpretation; and drafting and critical revision of the manuscript. All authors provided final approval and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval
The studies involving human participants were reviewed and approved by Ethics Committee of Shenzhen Children’s Hospital (No.201804802). All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional. Due to all participants included in the study are under 18, informed consent was obtained from all individual participants’ parents and/or legal guardian.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xian, Zx., Wang, X., Chen, Yc. et al. Preliminary assessment of portable sleep monitoring for diagnosis of obstructive sleep apnea in children. Sleep Breath 28, 419–425 (2024). https://doi.org/10.1007/s11325-023-02919-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-023-02919-9