Abstract
Purpose
Monitored polysomnography (PSG) is considered the gold standard technique to diagnose obstructive sleep apnea (OSA) and titrate continuous positive airway pressure (CPAP), the accepted primary treatment method. Currently, the American Academy of Sleep Medicine (AASM) considers automatic PAP therapy initiation at home comparable to laboratory titration and recommends telemonitoring-guided interventions. Advanced CPAP devices evaluate and report the residual apnea–hypopnea index (AHI). However, in order to control the effectiveness of the prescribed therapy outside of a PSG setting, the automatic event detection must provide reliable data.
Methods
A CPAP titration was performed in the sleep laboratory by PSG in patients with OSA. The residual event indices detected by the tested device (prismaLine, Loewenstein Medical Technology) were compared to the manually scored PSG indices. Results of the device (AHIFLOW) were compared according to the AASM scoring criteria 1A (AHI1A, hypopneas with a flow signal reduction of ≥ 30% with ≥ 3% oxygen reduction and/or an arousal) and 1B (AHI1B, hypopneas with a flow signal decrease by ≥ 30% with a ≥ 4% oxygen desaturation).
Results
In 50 patients with OSA, the mean PSG AHI1A was 10.5 ± 13.8/h and the PSG AHI1B was 7.4 ± 12.6/h compared to a mean device AHIFlow of 8.4 ± 10.0/h. The correlation coefficient regarding PSG AHI1A and AHIFlow was 0.968. The correlation regarding central hypopneas on the other hand was 0.153. There were few central events to be compared in this patient group.
Conclusion
The device-based analysis showed a high correlation in the determination of residual obstructive AHI under therapy. The recorded residual respiratory event indices in combination with the data about leakage and adherence of the studied device provide reliable information for the implementation and follow-up of CPAP therapy in a typical group of patients with OSA.
Trial Registration Number: ClinicalTrials.gov Identifier: NCT04407949, May 29, 2020, retrospectively registered.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) is a heterogeneous disease with great importance for public health. Untreated, it is associated with numerous significant comorbidities, symptoms, and health consequences [1,2,3,4,5,6]. Studies indicate OSA as an underdiagnosed disease with increasing prevalence and clinical and public health interest [7,8,9,10,11,12]. The morbidity of OSA highly depends on the disease severity, which is conventionally determined by the apnea–hypopnea-index (AHI), the number of respiratory events per hour of sleep [13]. Nocturnal continuous positive airway pressure (CPAP) therapy is accepted as a primary treatment for moderate to severe OSA and has a positive effect on mortality [14]. Still, therapy initiation and follow-up needs to be controlled. To meet the high demand for OSA testing under therapy, it is necessary to exploit cost-effective and reliable systems.
While the current American Association of Sleep Medicine (AASM) recommendations suggest a polysomnography (PSG) as diagnostic test to assess the grade of severity [13], the current recommendations also consider therapy initiation using autotitrating PAP (APAP) at home, and telemonitoring-guided interventions during the initial period of PAP therapy [15]. A current meta-analysis demonstrated equivalent effects on patient outcomes and no difference in residual OSA severity after the initiation of PAP at home compared to an in-laboratory titration. Few studies actually compared the device AHI with a full polysomnography [16, 17].
The data provided by the devices can be used as a reference for therapy adherence (usage time) and effectiveness (residual AHI), but the devices can also be adjusted and optimized remotely, using their built-in systems. Moreover, sleep-disordered breathing (SDB) is a chronic disease that requires regular checkups. Some countries already use the transmitted data to control CPAP therapy adherence. Yet, the health care and data protection regulations require clear responsibility management [18]. Reliability of the data and its use for patient outcome is key, especially when the telemedicine approaches escalate to further ventilation therapies with more advanced devices for home non-invasive mechanical ventilation (NIV) in other respiratory diseases [19].
The current PAP devices detect and record usage time, leakages, and residual respiratory events including flow limitations (FL), hypopneas, and apneas. Integrated sensors continuously monitor pressure changes, airflow changes, and vibrations during therapy to identify any residual SDB. When operating in automatic PAP mode, internal algorithms evaluate the signals to adjust the pressure as necessary. While in PSG, the more accurate sleep/wake detection allows to refer the number of events to the mere sleep time (total sleep time, TST), the PAP devices usually record and store the data throughout the entire time of operation (total recording time, TRT). Some devices might also register and remove artifacts (e.g., excessive air leakages) or wake periods. To address distinctions, a change in terminology for PAP-only recorded residual respiratory disorders into AHIFLOW has already been suggested by the American Thoracic Society (ATS) [20].
Since the devices of the different manufacturers present unique algorithms to detect residual SDB, it is important to determine the reliability of individual therapy devices and detection techniques against the gold standard PSG before important therapeutic decisions with concrete consequences for individual patients can be drawn from the device-based analyses in practice. The aim of this monocentric prospective study was to compare the recordings and analyses of the current generation of prismaLINE devices (Loewenstein Medical Technology, Germany) to a manual evaluation of a PSG in the sleep laboratory.
Methods
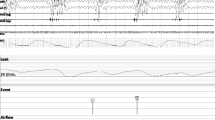
Inclusion criteria were age ≥ 18, moderate to severe sleep apnea syndrome (defined as AHI ≥ 15 n/h), recently diagnosed by PSG, indication for CPAP therapy, and use of a nasal mask. Exclusion criteria: CPAP contraindication, participation in another study that influences the setting of CPAP therapy by specifications regarding device setting or deviating titration needs. Patients needing an oronasal mask (OM) were excluded to ensure comparability. An acceptable study was one with a recorded TST > 4 h and < 10% of excessive leakage. During critically high leakage, there is a limited device response and possibly therapy ineffectiveness. From a leakage value of ≥ 50 L per minute, the “High leakage” event is recorded in the therapy device. The Ethics Committee of Witten-Herdecke University, Germany approved the study under number 192/2018. The trial was retrospectively registered under ClinicalTrials.gov Identifier: NCT04407949. Preliminary results were previously presented at the European Respiratory Society Congress in 2020 [21]. All patients provided consent to participate in the study after oral and written information about the study. Anthropometric baseline data were recorded at hospital admission. The cardiorespiratory PSG nights were performed in the certified sleep laboratory under the supervision of a sleep technician. The study-relevant therapy night with CPAP therapy under PSG took place immediately after the diagnosis night. The pressure titration procedure was predetermined for the study (see Fig. 1). Pressure started at 4 cm H2O, a sub therapeutic phase that allowed more residual events, and was increased every hour after sleep onset up to a pressure of 13 cm H2O, when sleep duration allowed this procedure.
Manual event detection (polysomnography)
The approved AASM guidelines version 2.4 [13] were used to define sleep and respiratory events for both diagnostic and therapeutic PSG recordings. The relevant flow signal was the nasal pressure signal in the diagnostic night, and the PAP device flow signal, which was fed into the PSG recording, in the titration study, as per recommendations of the AASM [13].
Recommended: According to the AASM guidelines, respiratory events during sleep were considered apneas if the peak airflow decreased by ≥ 90% for at least 10 s, and hypopneas in case of a flow signal reduction of at least 10 s of ≥ 30% of the baseline flow associated with a ≥ 3% oxygen reduction and/or an arousal (hypopnea criterion 1A).
Acceptable: In a secondary analysis, we scored respiratory events as hypopneas according to the acceptable AASM criterion 1B, when the flow signal decreased by ≥ 30% for at least 10 s, and this event was associated with a ≥ 4% oxygen desaturation from the pre-event baseline values.
Automatic event detection (therapy device)
The prismaLine type WM100TD CPAP device from Loewenstein Medical Technology is a CE-approved Class IIa medical device for the positive pressure treatment of sleep-related breathing disorders (PAP therapy). All used devices were of the same series and technically equivalent. The algorithm of the devices identifies residual AHI-relevant respiratory events (obstructive and central apneas and hypopneas) and other less severe events such as RERAflow (respiratory effort related arousal detected by the device), flow limitations, and snoring, also to control the auto-CPAP or auto-EPAP function [22]. The devices recognize a respiratory event as apnea if there is a respiratory flow interruption of at least 90% for at least 10 s.
Technique: An apnea is tested for possible upper airway obstruction by a 5 Hz FOT (forced oscillation technique) signal. If the oscillation is found mainly in the pressure signal, the current apnea is assessed as obstructive apnea. If the device detects the oscillation in the flow signal, the current apnea is categorized as central apnea. Hypopneas on the other hand are categorized as such, when there is a reduction in the respiratory flow curve of at least 50% for at least 10 s and two breaths. The current device class also differentiates hypopneas into central and obstructive. Mild obstructions during spontaneous breathing are detected by the degree of “flattening” in the inspiratory flow curve or by snoring measurement deduced from the flow curve. If this is detected during a hypopnea, it is classified as obstructive, otherwise as central.
Data comparison and statistical considerations
The evaluated PSG data as well as the recorded raw data of the devices were anonymized and evaluated separately in order to perform crosschecks. The scorers were blind to the CPAP evaluation. In order to achieve a sufficiently large number of relevant residual respiratory events in total, a number of 50 evaluable recordings were deemed to be necessary. This sample size made an evaluation in accordance with the methods of descriptive statistics reasonable.
The respiratory event indices detected by the devices were compared to the manually scored PSG event indices over the entire recording time of the device (AHIFlow) similar to daily routine in the sleep lab. Yet the data set, consisting of the scored PSG file and the device file, was synchronized by means of a cross correlation function. Periods without data overlap, i.e., when only one of the two devices recorded data, were cut out, and the events occurring in these periods were not considered. This applied to either the beginning or the end of the recordings, in case one of the recorders (device or PSG) ran longer or shorter than the other.
Data are presented as mean ± standard error as either variable or numbers in percent. Bland–Altmann-plot was chosen as the statistical method to visualize the deviation and agreement. The specificity, sensitivity, and positive and negative predictive value for the given device limits were calculated and plotted in receiver operating characteristics (ROC) curves.
Results
Seventy patients were recruited for the study. Twenty (29%) recordings were deemed unacceptable for final analysis (8 technical PSG issues with major artifacts in flow or neurologic leads, 6 human errors (wrong device or titration), 5 patients did not meet the required minimal TST, and 1 presented unsolvable mask problems with excessive leakage). Fifty patients (female: 13; age: 55.1 ± 11.9 years; body mass index: 35.5 ± 7.4 kg/m2) were included in the final analysis. Baseline AHI was 53.3 ± 24.2. Three patients had a history of coronary heart disease, and 10 patients showed a baseline central AI > 5. Mean baseline AI was 3.4 ± 5.3 (central apnea (n) were 19.8 ± 30.9).
We obtained a mean manually evaluated PSG AHI of 10.5 ± 13.8/h according to criterion 1A and 7.4 ± 12.6/h according to criterion 1B compared to a mean device AHIFlow of 8.4 ± 10.0/h (p = 0.004). All results and correlations are presented in Table 1. The positive and negative predictive value for an AHI > 10, scored according to criterion 1A, were 0.909 and 0.795.
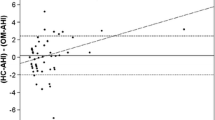
The Bland–Altman plots visualize the difference between the total AHI-PSG according to criterion 1A and 1B, respectively (manually assessed events minus events detected by the device) plotted against their respective average values (see Figs. 2 and 3). Overall, there is little variation in the deviation. The ROC curves describe the quality of the test procedure in which it plots sensitivity (true positive rate) against false positive rate (1-specificity). In our case, the PSG was the standard against which the device AHI (AHIFlow) was measured (see Fig. 4). The two cut off values of AHI (AHI5 and AHI10) refer to the state “still sick” and “healthy.” A pair of sensitivity/specificity was calculated for the whole list of values (device AHIFlow), respectively, and plotted as a curve. The area between the curve and the angle bisector (AUC) can assume values of 0.5–1. Sensitivity and specificity results are presented in Table 2.
Discussion
The data collected in this study demonstrated a high correlation in the determination of the residual apnea and hypopnea indices between the CPAP therapy device and a simultaneous PSG in patients with moderate to severe obstructive sleep apnea. To our knowledge, this is the first study that also examines the influence of AASM criteria 1A and 1B. Compared to the evaluation according to AASM criterion 1A, the device results were only slightly lower, and compared to the evaluation according to 1B, slightly higher. The relevant flow signal for the therapy night was the CPAP flow signal, as per current AASM recommendations. (14) A PSG specific flow signal could have led to other results. The AASM recommended to routinely use the device flow in 2012, but some subsequent studies found different results when using a PSG flow signal versus the device flow signal of an APAP machine during titration [23, 24]. The discrepancies were found in the absolute agreement of the respiratory event classification in spite of good diagnostic accuracy concerning the AHI results. That results relate to older devices of another manufacturer and older PSG systems, it is nevertheless reasonable to assume, that close clinical follow-up is necessary for patients with complex SDB (e.g., high central apnea or high arousal), or if response to treatment is poor.
Other studies on PAP devices that compared the respiratory event detection found the strongest correlations in the detection of obstructive events [25, 26]. In our study, we also found only minor deviations concerning central events, despite the fact, that the PAP devices’ evaluation of residual SDB must rely on flow/pressure analysis alone. While only a limited number of central events were recorded to compare in this predominantly obstructive sleep apnea group, it indicates a sufficient comparability for the standard patient with OSA. A PSG recording, on the other hand, provides further bio signals such as respiratory effort (thorax and abdomen belts), pulse oximetry, electrocardiogram (ECG), brain waves by electroencephalogram (EEG), eye movements by electrooculogram (EOG), and muscle tone by electromyogram (EMG). When analyzing a PSG, the recording of respiratory effort helps to distinguish apnea types, while the neurologic leads allow a differentiation of wakefulness and sleep and an evaluation of different sleep stages.
There are only a few studies that have compared respiratory event detection algorithms from PAP devices with manually assessed events obtained from PSG. Ueno et al. and Berry compared a PAP device with PSG and found higher AHI values at lower pressure levels compared with the PSG and lower AHI values at higher pressure levels. In summary, an AHI < 10/h of the studied PAP device was highly predictive, but an AHI > 10/h was moderately predictive. The event-by-event analysis showed that the automatic event detection had a high specificity but only modest sensitivity (high number of false negatives). The correlation of hypopneas, however, was rather low. The software was also unable to distinguish central apneas [25, 27].
In our study, the CPAP device showed the highest sensitivity for a threshold level for the AHI between 5 and 10/h. When comparing the indices of the PSG 1A and 1B evaluations, a lower sensitivity resulted (see Table 2). A lesser sensitive scoring rule might have a negative impact, when events remain undetected and residual symptoms unexplained. On the other hand, the more sensitive rule may lead to the prescription of a higher therapy pressure with an effect on adherence. A device AHI, present in between these two, might actually be favorable. The lowest correlations were found with regard to central hypopneas, whereas there were only a few events of that type to be compared in our patient population. Future study could focus on patients with more complex and central sleep apnea.
Previous studies showed a high percentage of patients with presumed good PAP therapy success, but still considerable residual respiratory events [28, 29]. Reliable data with regard to the residual event indices are of clinical importance, but patient-reported daytime sleepiness and relevant comorbidities must be included when assessing clinical outcomes [30]. Controlled PAP recordings may not only provide an explanation for possible residual symptoms but may also contribute to the decision to readmit the patient to a sleep center for a new titration.
The COVID-19 pandemic imposed a challenge to sleep laboratories and patients with OSA worldwide. On the one hand, the necessary social distancing measures and the fear of contamination kept patients with non-acute medical conditions from going to the sleep lab, and on the other hand, the need to reschedule ward capacities with temporary and possibly longer-term closures of sleep lab units dramatically reduced sleep medicine services. Regional surveys as well as international studies researched the situation during the pandemic [31]. In Europe, in-lab PSG was reduced from 93 to 20%, and 72% of sleep centers stopped in-lab PAP titration. A third of the European sleep centers started to implement telemedicine services [32]. In the USA and Canada, in-lab testing was reduced by 90% [33], as also in China, where the sleep medicine services recovered to 50% from baseline after the first wave [34].
With certainty, there are numerous aspects of the diagnostic process of OSA that require polysomnographic surveillance. The association of respiratory events, body position, sleep stages, or the extent of nocturnal hypoxia is useful in assessing the disease severity and the underlying physiological causes. A possible strategy to improve the detection sensitivity of a CPAP device could be the integration of oximetry, most preferably in a wireless setting. While the prevalence of SDB increases and sleep center capacities are being reduced, new methods for the diagnosis and treatment control are developing. Cost efficient new technologies are demanded, and telemedicine approaches are implemented, which allow diagnosis and surveillance with minimal patient interface [35].
The high correlation of the respiratory event detection between the tested CPAP device and the information from all bio-signals of the PSG in our study indicates that analysis of respiration alone may be a sufficient risk assessment with CPAP therapy. But patient reported side effects, e.g., via questionnaires, will also play a major role when assessing therapy efficacy and effectiveness by telemedicine, especially the opportunity to record and monitor the residual events with adequate precision over extended periods of time promises to have a positive effect on CPAP-treated patients. Given the night-by-night variability of AHI in a PSG [36], a long-term serial measurement of AHIFlow with a reliable PAP device may actually be favorable.
Limitations
Our study population consisted of predominantly male, obese patients with moderate- to severe disease and reflects a typical population with OSA. Therefore, there is at most a small selection bias. The recordings took place under optimal laboratory conditions. The findings must therefore be translated to the outpatient setting with caution. The study was conducted using the device of Loewenstein Medical Technology with its proprietary technology and the results do not inevitably apply to devices from other companies. This could be the subject of further studies. Patients with high leakage (> 10% of excessive leakage of > 40 L/min) were excluded, as well as oronasal masks (OM). The mask choice makes results more comparable, and generally, nasal masks should always be the first choice and are the most used interfaces. Yet, 28% of the patients of a recent large study on mask side effects were fitted with an OM [37]. Patients titrated with oronasal masks require higher pressure levels and present higher leak and higher residual AHI when compared to nasal masks [38]. It remains unclear, to what extent the results would have been influenced by a subgroup with OM and presumably higher leakages. We chose to exclude patients needing OM in this study, because the presumed subgroup would have been too small to present reliable statistics. It would be reasonable to study this patient group separately.
Our study design did not allow for evaluation at higher pressure levels than 13 cm H2O, yet, such pressures are uncommonly applied. Further studies are needed to verify the diagnostic agreement in settings where higher pressure are required. Whether or not and to what extent any comfort features like pressure relief, an oronasal mask, or even the frequently used humidifier may have an effect on event detection was not part of this research.
Conclusion
The results of this study demonstrated a high correlation in the determination of residual sleep apnea derived from a CPAP device compared with a polysomnography in a typical population with OSA. The reliable information about residual events, along with leakage and usage time, may be available for telemedicine applications. However, symptom health assessment should always be included in therapy decisions. The agreement of PSG findings and device findings should be investigated separately for different CPAP devices and the different algorithms used by manufacturers.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AASM :
-
American Association of Sleep Medicine
- AHI :
-
Apnea–hypopnea index
- AI :
-
Apnea index
- APAP :
-
Autotitrating positive airway pressure
- AUC :
-
Area under the curve
- BMI :
-
Body mass index
- cAHI :
-
Central apnea hypopnea index
- cHI :
-
Central hypopnea index
- cAI :
-
Central apnea index
- cmH2O :
-
Centimeters of water column
- COVID :
-
Coronavirus disease
- CPAP :
-
Continuous positive airway pressure
- ECG :
-
Electrocardiogram
- EEG :
-
Electroencephalogram
- EMG :
-
Electromyogram
- EOG :
-
Electrooculogram
- EPAP :
-
Expiratory positive airway pressure
- FL :
-
Flow limitations
- FOT :
-
Forced oscillation technique
- HI :
-
Hypopnea index
- HSAT :
-
Home sleep apnea testing
- NIV :
-
Non-invasive ventilation
- oAI :
-
Obstructive apnea index
- oHI :
-
Obstructive hypopnea index
- oAHI :
-
Obstructive apnea hypopnea index
- OSA :
-
Obstructive sleep apnea
- PAP :
-
Positive airway pressure
- PSG :
-
Polysomnography
- RERA :
-
Respiratory effort-related arousal
- ROC :
-
Receiver operating characteristics
- SDB :
-
Sleep-disordered breathing
- TRT :
-
Total run time
- TST :
-
Total sleep time
References
Pinto JA, Ribeiro DK, Cavallini AF, Duarte C, Freitas GS (2016) Comorbidities associated with obstructive sleep apnea: a retrospective study. Int Arch Otorhinolaryngoly 20(2):145–150. https://doi.org/10.1055/s-0036-1579546
Osman AM, Carter SG, Carberry JC, Eckert D (2018) Obstructive sleep apnea: current perspectives. Nat Sci Sleep 10:21–34. https://doi.org/10.2147/NSS.S124657 (Published 2018 Jan 23)
Marin JM, Agusti A, Villar I et al (2012) Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 307(20):2169–2176. https://doi.org/10.1001/jama.2012.3418
Gottlieb DJ, Yenokyan G, Newman AB et al (2010) Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 122(4):352–360. https://doi.org/10.1161/CIRCULATIONAHA.109.901801
Weaver TE, Maislin G, Dinges DF et al (2007) Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 30(6):711–719. https://doi.org/10.1093/sleep/30.6.711
Deegan PC, McNicholas WT (1995) Pathophysiology of obstructive sleep apnoea. Eur Respir J 8(7):1161–1178. https://doi.org/10.1183/09031936.95.08071161
Senaratna CV, Perret JL, Lodge CJ et al (2017) Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 34:70–81. https://doi.org/10.1016/j.smrv.2016.07.002
Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J (2002) Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 6(2):49–54. https://doi.org/10.1007/s11325-002-0049-5
The Lancet Respiratory Medicine (2020) Time to wake the giant of obstructive sleep apnoea. Lancet Respir Med 8(1):1. https://doi.org/10.1016/S2213-2600(19)30449-7
Peppard PE, Young T, Barnet JH et al (2013) Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177(9):1006–1014. https://doi.org/10.1093/aje/kws342.10.1093/aje/kws342
Young T, Palta M, Dempsey J et al (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328(17):1230–1235. https://doi.org/10.1056/NEJM199304293281704
Heinzer R, Vat S, Marques-Vidal P et al (2015) Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 3(4):310–318. https://doi.org/10.1016/S2213-2600(15)00043-0
Berry RB, Brooks R, Gamaldo CE, for the American Academy of Sleep Medicine et al (2017) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Darien, IL: American Academy of Sleep Medicine; Version 2.4. J Clin Sleep Med 13(5):665–666. https://doi.org/10.5664/jcsm.6576
Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG (2019) Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 15(2):335–343. https://doi.org/10.5664/jcsm.7640 (Published 2019 Feb 15)
Singh J, Badr MS, Diebert W et al (2015) American Academy of Sleep Medicine (AASM) position paper for the use of telemedicine for the diagnosis and treatment of sleep disorders. J Clin Sleep Med 11(10):1187–1198. https://doi.org/10.5664/jcsm.5098 (Published 2015 Oct 15)
Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG (2019) Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J Clin Sleep Med 15(2):301–334. https://doi.org/10.5664/jcsm.7638 (Published 2019 Feb 15)
Bertelli F, Suehs CM, Mallet JP et al (2021) Apnoea-hypopnoea indices determined via continuous positive airway pressure (AHI-CPAPflow) versus those determined by polysomnography (AHI-PSGgold): a protocol for a systematic review and meta-analysis. BMJ Open 11(5):e044499. https://doi.org/10.1136/bmjopen-2020-044499 (Published 2021 May 10)
Randerath W, Bögel M, Franke C, Hellmann A, Jany B, Nilius G, Penzel T, Voshaar T, Wiater A (2017) Positionspapier zum Telemonitoring bei schlafbezogenen Atmungsstörungen [Positionpaper on Telemonitoring in Sleep-Related Breathing Disorders]. Pneumologie (Stuttgart Germany) 71(2):81–85. https://doi.org/10.1055/s-0042-124083
Borel JC, Palot A, Patout M (2019) Technological advances in home non-invasive ventilation monitoring: reliability of data and effect on patient outcomes. Respirology 24(12):1143–1151. https://doi.org/10.1111/resp.13497
Schwab RJ, Badr SM, Epstein LJ et al (2013) An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med 188(5):613–620. https://doi.org/10.1164/rccm.201307-1282ST
Nilius G, Schroeder M, Domanski U, Richter M (2020) A comparison of sleep disordered breathing events detected by CPAP devices with in-lab polysomnography. Eur Respir J 56:2491. https://doi.org/10.1183/13993003.congress-2020.2491
Herkenrath SD, Treml M, Anduleit N et al (2020) Extended evaluation of the efficacy of a proactive forced oscillation technique-based auto-CPAP algorithm. Sleep Breath 24(3):825–833. https://doi.org/10.1007/s11325-019-01901-8
Huang HC, Hillman DR, McArdle N (2012) Control of OSA during automatic positive airway pressure titration in a clinical case series: predictors and accuracy of device download data. Sleep 35(9):1277–83A. https://doi.org/10.5665/sleep.2086 (Published 2012 Sep 1)
Nigro CA, González S, Arce A, Aragone MR, Nigro L (2015) Accuracy of a novel auto-CPAP device to evaluate the residual apnea-hypopnea index in patients with obstructive sleep apnea. Sleep Breath 19(2):569–578. https://doi.org/10.1007/s11325-014-1048-z
Berry RB, Kushida CA, Kryger MH, Soto-Calderon H, Staley B, Kuna ST (2012) Respiratory event detection by a positive airway pressure device. Sleep 35(3):361–367. https://doi.org/10.5665/sleep.1696 (Published 2012 Mar 1)
Ikeda Y, Kasai T, Kawana F et al (2012) Comparison between the apnea-hypopnea indices determined by the REMstar Auto M series and those determined by standard in-laboratory polysomnography in patients with obstructive sleep apnea. Intern Med 51(20):2877–2885. https://doi.org/10.2169/internalmedicine.51.8249
Ueno K, Kasai T, Brewer G et al (2010) Evaluation of the apnea-hypopnea index determined by the S8 auto-CPAP, a continuous positive airway pressure device, in patients with obstructive sleep apnea-hypopnea syndrome. J Clin Sleep Med 6(2):146–151
Pittman SD, Pillar G, Berry RB, Malhotra A, MacDonald MM, White DP (2006) Follow-up assessment of CPAP efficacy in patients with obstructive sleep apnea using an ambulatory device based on peripheral arterial tonometry. Sleep Breath 10(3):123–131. https://doi.org/10.1007/s11325-006-0058-x
Baltzan MA, Kassissia I, Elkholi O, Palayew M, Dabrusin R, Wolkove N (2006) Prevalence of persistent sleep apnea in patients treated with continuous positive airway pressure. Sleep 29(4):557–563
Rotty MC, Suehs CM, Mallet JP et al (2021) Mask side-effects in long-term CPAP-patients impact adherence and sleepiness: the InterfaceVent real-life study. Respir Res 22(1):17. https://doi.org/10.1186/s12931-021-01618-x
Grote L, Theorell-Haglöw J, Ulander M, Hedner J (2021) Prolonged effects of the COVID-19 pandemic on sleep medicine services-longitudinal data from the Swedish Sleep Apnea Registry. Sleep Med Clin 16(3):409–416. https://doi.org/10.1016/j.jsmc.2021.05.008
Grote L, McNicholas WT, Hedner J, ESADA collaborators (2020) Sleep apnoea management in Europe during the COVID-19 pandemic: data from the European Sleep Apnoea Database (ESADA). Eur Respir J 55(6):2001323. https://doi.org/10.1183/13993003.01323-2020 (Published 2020 Jun 18)
Johnson KG, Sullivan SS, Nti A et al (2021) The impact of the COVID-19 pandemic on sleep medicine practices. J Clin Sleep Med 17(1):79–87. https://doi.org/10.5664/jcsm.8830
Zhang XL, Wang W, Xiao Y (2021) Members of the assembly of sleep disordered breathing of the Chinese thoracic society. Sleep disordered breathing diagnosis and treatment during the COVID-19 pandemic: a nationwide survey in China. Nat Sci Sleep 13:21–30. https://doi.org/10.2147/NSS.S292373
Penzel T, Schöbel C, Fietze I (n.d.) New technology to assess sleep apnea: wearables, smartphones, and accessories. F1000Res 7:413. Published 2018 Mar 29. https://doi.org/10.12688/f1000research.13010.1
Roeder M, Bradicich M, Schwarz EI et al (2020) Night-to-night variability of respiratory events in obstructive sleep apnoea: a systematic review and meta-analysis. Thorax 75(12):1095–1102. https://doi.org/10.1136/thoraxjnl-2020-214544
Rotty MC, Suehs CM, Mallet JP et al (2021) Mask side-effects in long-term CPAP-patients impact adherence and sleepiness: the InterfaceVent real-life study. Respir Res 22(1):17. https://doi.org/10.1186/s12931-021-01618-x (Published 2021 Jan 15)
Rowland S, Aiyappan V, Hennessy C et al (2018) Comparing the efficacy, mask leak, patient adherence, and patient preference of three different CPAP interfaces to treat moderate-severe obstructive sleep apnea. J Clin Sleep Med 14:101–108. https://doi.org/10.5664/jcsm.6892
Acknowledgements
The authors would like to thank Regina Schaefer, Alexander Grimm, Jan Verhoeven, and Ruediger Alshut for their help in evaluating, analyzing, and interpreting the recorded data.
Funding
Loewenstein Medical Technology provided financial support for the study. This has gone into departments funds. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
All authors have seen and approved the manuscript. Matthias Richter: draft of the article, analysis and interpretation of data, critical review, and final approval. M Schroeder, U Domanski, M Schwaibold, and G Nilius: analysis and interpretation of data, critical review, draft correction and proof reading, and final approval.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the ethics committee of Witten/Herdecke University under number 192/2018 and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
Conflict of interest
M Richter, M Schroeder, and U Domanski have no financial or other potential conflicts of interest associated with this study. M Schwaibold is employee of Loewenstein Medical Technology. G Nilius has received research support from Loewenstein Medical Technology, Fisher & Paykel Healthcare, ResMed and Weinmann; this has gone into department funds.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Richter, M., Schroeder, M., Domanski, U. et al. Reliability of respiratory event detection with continuous positive airway pressure in moderate to severe obstructive sleep apnea — comparison of polysomnography with a device-based analysis. Sleep Breath 27, 1639–1650 (2023). https://doi.org/10.1007/s11325-022-02740-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-022-02740-w