Abstract
Purpose
Obstructive sleep apnea (OSA) is prevalent in the bariatric population. OSA should be recognized in patients undergoing bariatric surgery preoperatively to prevent peri- and post-operative complications. Lipid metabolism-related biomarkers are associated with OSA. Triglyceride metabolism is, among others, regulated by angiopoietin-like protein five (ANGPTL5). We aimed to evaluate the level of ANGPTL5 in patients with OSA of different severity levels before and after bariatric surgery.
Methods
We performed a single-center prospective cohort study including a consecutive series of patients who underwent bariatric surgery. We collected the clinical data, polysomnography (PSG) or polygraphy (PG) parameters, and plasma derived via venipuncture before and 6 to 12 months after surgery. Lipid profile, glucose levels, and ANGPTL5 levels were assessed. ANGPTL5 levels were measured using an enzyme-linked immunosorbent assay (ELISA).
Results
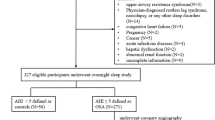
The study included 88 patients for analysis. The patients were divided into two subgroups: no or mild OSA (apnea–hypopnea index (AHI) < 15 events/hour, n = 57) and moderate-to-severe OSA (AHI ≥ 15 events/hour, n = 31). The ANGPTL5 level was higher in the moderate-to-severe OSA group (20.5 [15.6, 26.5] ng/mL) compared to the no or mild OSA group (16.3 [12.5, 19.4] ng/mL) (p = 0.008). A significant positive correlation was observed between ANGPTL5 and AHI (ρ = 0.256, p = 0.017), apnea index (AI) (ρ = 0.318, p = 0.003), and triglyceride levels (ρ = 0.240, p = 0.025). ANGPTL5 levels were reduced significantly after bariatric surgery in both moderate-to-severe OSA (15.6 [10.3, 18.7] ng/mL) and no or mild OSA (13.4 [9.2, 15.8] ng/mL) groups, though to a lower level in the group without or mild OSA. Post-surgery, the significant positive correlation between ANGPTL5 and AHI (ρ = 0.210, p = 0.047), AI (ρ = 0.230, p = 0.034), and triglyceride (ρ = 0.397, p < 0.001) remained.
Conclusion
The data showed increased levels of ANGPTL5 in patients with moderate-to-severe OSA. Both AHI and ANGPTL5 levels decreased significantly after bariatric surgery. We also report an association between ANGPTL5 levels and OSA severity.






Similar content being viewed by others
Abbreviations
- AHI:
-
Apnea-hypopnea index
- AI:
-
Apnea index
- ANGPTL:
-
Angiopoietin-like protein
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CPAP:
-
Continuous positive airway pressure
- CVD:
-
Cardiovascular disease
- DDI:
-
Dasman Diabetes Institute
- ELISA:
-
Enzyme-linked immunosorbent assay
- HbA1C:
-
Glycosylated haemoglobin, type A1C
- HDL:
-
High-density lipoprotein
- HI:
-
Hypopnea index
- HIF:
-
Hypoxemia-inducible factor 1
- HsCRP:
-
High sensitivity C-reactive protein
- LDL:
-
Low-density lipoprotein
- LPL:
-
Lipoprotein lipase
- LRYGB:
-
Laparoscopic Roux-en-Y gastric bypass
- LSG:
-
Laparoscopic sleeve gastrectomy
- OSA:
-
Obstructive sleep apnea
- PG:
-
Polygraphy
- PSG:
-
Polysomnography
- ROC:
-
Receiver Operating Characteristic
- T2D:
-
Type 2 diabetes
- TGL:
-
Triglyceride
References
Lam JC, Mak JC, Ip MS (2012) Obesity, obstructive sleep apnoea and metabolic syndrome. Respirology 17(2):223–236
Sanchez-de-la-Torre M, Campos-Rodriguez F, Barbe F (2013) Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med 1(1):61–72
de Raaff CAL, de Vries N, van Wagensveld BA (n.d) Obstructive sleep apnea and bariatric surgical guidelines: summary and update. (1473–6500 (Electronic)).
Dempsey JA et al (2010) Pathophysiology of sleep apnea. Physiol Rev 90(1):47–112
Angrisani L et al (2017) Bariatric surgery and endoluminal procedures: IFSO Worldwide Survey 2014. Obes Surg 27(9):2279–2289
Angrisani L et al (2021) Bariatric surgery survey 2018: similarities and disparities among the 5 IFSO chapters. Obes Surg 31(5):1937–1948
Epstein LJ et al (2009) Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 5(3):263–276
Butt M et al (2010) Obstructive sleep apnea and cardiovascular disease. Int J Cardiol 139(1):7–16
Gopalakrishnan P, Tak T (2011) Obstructive sleep apnea and cardiovascular disease. Cardiol Rev 19(6):279–290
Rasche K et al (2010) Obstructive sleep apnea and type 2 diabetes. Eur J Med Res 15(Suppl 2):152–156
Kendzerska T et al (2014) Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med 190(2):218–25
Godoy J et al (2009) Obstructive sleep apnea as an independent stroke risk factor: possible mechanisms. Curr Mol Med 9(2):203–209
Ryan S (2017) Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J Physiol 595(8):2423–2430
Passali D et al (2015) Oxidative stress in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital 35(6):420–425
Lin MT et al (2015) Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a meta-analysis. Sleep Breath 19(3):809–817
Jun JC et al (2012) Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab 303(3):E377–E388
Drager LF et al (2012) Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J 33(6):783–790
Yao Q et al (2013) Effect of chronic intermittent hypoxia on triglyceride uptake in different tissues. J Lipid Res 54(4):1058–1065
Li J et al (2020) Triglyceride metabolism and angiopoietin-like proteins in lipoprotein lipase regulation. Clin Chim Acta 503:19–34
Abu-Farha M et al (2020) The multi-faces of Angptl8 in health and disease: novel functions beyond lipoprotein lipase modulation. Prog Lipid Res 80:101067
Santulli G (2014) Angiopoietin-like proteins: a comprehensive look. Front Endocrinol 5:4
Carbone C et al (2018) Angiopoietin-like proteins in angiogenesis, inflammation and cancer. Int J Mol Sci 19(2): https://doi.org/10.3390/ijms19020431.
Miida T, Hirayama S (2010) Impacts of angiopoietin-like proteins on lipoprotein metabolism and cardiovascular events. Curr Opin Lipidol 21(1):70
Zeng L et al (2003) Identification of a novel human angiopoietin-like gene expressed mainly in heart. 48:159.
Romeo S et al (2009) Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Investig 119(1):70
Hammad MM et al (2020) Correlation of circulating ANGPTL5 levels with obesity, high sensitivity C-reactive protein and oxidized low-density lipoprotein in adolescents. Sci Rep 10(1):6330
Alghanim G et al (2019) Higher levels of ANGPTL5 in the circulation of subjects with obesity and type 2 diabetes are associated with insulin resistance. Front Endocrinol 10:495
Abu-Farha M, Abubaker J, Tuomilehto J (2017) ANGPTL8 (betatrophin) role in diabetes and metabolic diseases. Diabetes/Metab Res Rev 33(8): https://doi.org/10.1002/dmrr.2919
Li C (2007) A tale of two angiopoietin-like proteins. Curr Opin Lipidol 18(5):597
Mattijssen F, Kersten S (2012) Regulation of triglyceride metabolism by angiopoietin-like proteins. Biochem Biophys Acta 1821(5):782
Kersten S (2005) Regulation of lipid metabolism via angiopoietin-like proteins. Biochem Soc Trans 33(Pt 5):1059
Haller JF et al (2017) ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J Lipid Res 58(6):1166–1173
Leth-Espensen KZ et al (2021) The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding. Proc Natl Acad Sci 118(12):e2026650118
Wang Y et al (2013) Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc Natl Acad Sci U S A 110(40):16109–16114
Oteng AB et al (2019) Characterization of ANGPTL4 function in macrophages and adipocytes using Angptl4-knockout and Angptl4-hypomorphic mice. J Lipid Res 60(10):1741–1754
Köster A et al (n.d) Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. (0013–7227 (Print)).
Drager LF, Polotsky VY (2011) Lipid metabolism: a new frontier in sleep apnea research. Am J Respir Crit Care Med 184(3):288–290
Savransky V et al (2007) Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 175(12):1290–1297
Al-Terki A et al (2018) Increased level of angiopoietin like proteins 4 and 8 in people with sleep apnea. Front Endocrinol 9:651
Li J et al (2020) The clinical role of angiopoietin-like protein 3 in evaluating coronary artery disease in patients with obstructive sleep apnea. Cardiovasc Drugs Ther 34(6):773–780
Schauer PR et al (2012) Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366(17):1567–1576
Cho J-M et al (2015) Effect of sleeve gastrectomy on type 2 diabetes as an alternative treatment modality to Roux-en-Y gastric bypass: systemic review and meta-analysis. Surg Obes Relat Dis 11(6):1273–1280
Singh AK, Singh R, Kota SK (2015) Bariatric surgery and diabetes remission: who would have thought it? Indian J Endocrinol Metab 19(5):563–576
Singh P et al (2020) Impact of bariatric surgery on cardiovascular outcomes and mortality: a population-based cohort study. Br J Surg 107(4):432–442
Marin JM et al (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365(9464):1046–1053
Ban JJ et al (2014) Regulation of obesity and insulin resistance by hypoxia-inducible factors. Hypoxia (Auckl) 2:171–183
Bradley TD, Floras JS (2009) Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373(9657):82–93
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leentjens, M., Bosschieter, P.F.N., Al-Terki, A. et al. The association between biomarker angiopoietin-like protein five and obstructive sleep apnea in patients undergoing bariatric surgery. Sleep Breath 27, 1443–1454 (2023). https://doi.org/10.1007/s11325-022-02736-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-022-02736-6