Abstract
Purpose
The aim of this study was to analyze the relationship of snoring sound signals obtained by polysomnography (PSG) in the sleep laboratory with cortical EEG (6 channel) signals to find answers to two important questions that have been covered to a limited extent in the literature: (1) Would the sounds generated by a snoring individual have an effect on the cerebral electrical waves occurring during sleep (specifically deep restorative sleep)? (2) Would the snoring sounds of an individual being examined by PSG have more of an effect on any one of the EEG electrodes?
Methods
PSG recordings were obtained from volunteers with primary snoring and those with obstructive sleep apnea syndrome (OSAS) on six different EEG channels (F4-M1, C4-M1, and O2-M1, F3-M2, C3-M2, and O1-M2). The relationship of each of these recordings and snoring sound signals was analyzed by using a computer-based electrophysiological signal analysis method. A three-tier approach was used in this relationship: “Feature extraction, Feature selection, and Classification”.
Results
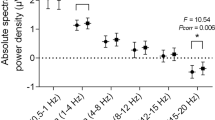
Data were obtained from a total of 40 volunteers (32 men, mean age (± SD) 47.5 ± 3.2 years), 20 with primary snoring and 20 with OSAS. The discrete wavelet transform (DWT) feature extraction method was the most successful method, and by utilizing this method for analyzing EEG channels, snoring sound signals were found to affect the C3-M2 channel the most (Duncan test, p < 0.05). Delta wave frequency levels during snoring were decreased compared to both before snoring (p = 0.160) and after snoring (p = 0.04) periods (paired sample test).
Discussion
When snoring sounds and EEG signals were analyzed for frequency, time, and wave conversion with feature extraction methods, the C3-M2 channel was to be found the most affected channel. The sleep physiologist who made the PSG analyses reported that, among the 6 EEG channels analyzed for periods where there was no apnea or hypopnea events but only snoring, C3-M2 was the channel showing changes in delta wave activity.
Conclusion
Our study showed that the monotonous and repetitive snoring sounds of the snorer do not wake the individual, but do affect deep restorative sleep (N3). PSG signal analysis revealed that the most significant changes were in the C3-M2 channel (N3 delta wave amplitude increase and frequency decrease during snoring). Thus, clinicians may be able to monitor the characteristic changes occuring in large cortical delta waves in snoring individuals with innovative single-channel EEG devices without microphones.






Similar content being viewed by others
References
Kayabekir M (2020) Diagnosis. In: Fabian HR (ed) Updates in sleep neurology and obstructive sleep apnea. First ed. London Bridge Street: Intechopen, pp 1–13
Kayabekir M (2019) Sleep physiology and polysomnogram, physiopathology and symptomatology in sleep medicine. In: Fabian HR (ed) Updates in Sleep Neurology and Obstructive Sleep Apnea. First ed. London Bridge Street: Intechopen, pp 5–12
Elmasry A, Lindberg E, Berne C, Janson C, Gislason T, Awad Tageldin MA, Boman G (2001) Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med 249:153–161
Young T, Peppard P (2000) Sleep-disordered breathing and cardiovascular disease: epidemiologic evidence for a relationship. Sleep 23:122–126
Stradling J, Crosby J (1991) Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle-aged men. Thorax 46:85–90
Ayappa I, Rapoport DM (2003) The upper airway in sleep: physiology of the pharynx. Sleep Med Rev 7:9–33
McCormick DA, Bal T (1994) Sensory gating mechanisms of the thalamus. Curr Opin Neurobiol 4:550–556
Cairns BE, McErlane SA, Fragoso MC, Jia WG, Soja PJ (1996) Spontaneous discharge and peripherally evoked orofacial responses of trigemino-thalamic tract neurons during wakefulness and sleep. J Neurosci 16:8149–8159
Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ (2002) Model of thalamocortical slow-wave sleep oscillations and transitions to activated states. J Neurosci 22:8691–8704
Bastuji H, Garcia-Larrea L, Franc C, Mauguiere F (1995) Brain processing of stimulus deviance during slow-wave and paradoxical sleep: a study of human auditory evoked responses using the oddball paradigm. J Clin Neurophysiol 12:155–167
Afifi L, Guilleminault C, Colrain IM (2003) Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir Physiol Neurobiol 136:221–234
Chirakalwasan N, Ruzicka DL, Burns JW (2013) Chervin RD. Do snoring sounds arouse the snorer? SLEEP 36(4):565–571
Bostner Z, Knoll G, Lindner B (2020) Information filtering by coincidence detection of synchronous population output: analytical approaches to the coherence function of a two-stage neural system. Biological Cybernetics 1–16
Strang G, Nguyen T (1996) Filters. Wavelets and filter banks. Wellesley Cambridge Press. pp 37–80
Eftekhar A, Vohra F, Toumazou C, Drakakis EM, Parker K (2008) Hilbert-Huang transform: preliminary studies in epilepsy and cardiac arrhythmias. Biomedical Circuits and Systems Conference IEEE pp 373–376
Luque J, Anguita D, Perez F, Denda R (2020) Spectral analysis of electricity demand using Hilbert-Huang transform. Sensors 20(10):2912
Heck M, Hobiger M, Van Herwijnen A, Schweizer J, Fäh D (2019) Localization of seismic events produced by avalanches using multiple signal classification. Geophys J Int 216(1):201–217
Sharma C, Ojha CSP (2020) Statistical parameters of hydrometeorological variables: standart deviation, SNR, skewness and kurtosis. In Advances in Water Resources Engineering and Management. In: R. Al Khaddrar et al (eds) First Ed. Springer Nature Singapore, pp: 59–70
Saini M, Satija U, Upadhayay MD (2020) Effective automated method for detection and suppression of muscle artefacts from single-channel EEG signal. Halthcare Tecnology Letters 7(2):35–40
Maragos P, Kaiser JF, Quatieri TF (1993) Energy separation in signal modulations with application to speech analysis. IEEE Transsections on signal processing 41(10):3024–3051
Martorano PP, Falzetti G, Pelaia P (2006) Bispectral index and spectral entropy in neuroanesthesia. J Neurosurg Anesthesiol 18(3):205–210
Hammer D, Romashchenko A, Shen A, Vereshchagin N (2000) Inequalities for Shannon entropy and Kolmogorov complexity. J Comput Syst Sci 60(2):442–464
Zhang J, Chen M, Zhao S, Hu S, Shi Z, Cao Y (2016) ReliefF-Based EEG sensor selection methods for emotion recognition. Sensors 16:1558
Yağanoğlu M, Köse C (2017) Wearable vibration based computer interaction and communication system for deaf. Appl Sci 7(12):1296
Bolón-Canedo V, Sánchez-Marono N, Alonso Betanzos A, Benıtez JM, Herrera FA (2014) Review of microarray datasets and applied feature selection methods. Inf Sci 282:111–135
Rosario SF, Thangadurai K (2015) RELIEF: feature selection approach. International journal of innovative research and development 4(11):218–224
Nikitakis A, Makantasis K, Tampouratzis N, Papaefstathiou I (2019) A unified novel neural network approach and a prototype hardware implementation for Ultra-Low Power EEG classification. IEEE Trans Biomed Circuits Syst 13(4):670–681
Wang X, Gong G, Li N, Qiu S (2019) Detection analysis of epileptic EEG using a novel Random Forest Model combined with grid search optimization. Front Hum Neurosci 13(52):1–13
Zhang T, Chen W, Li M (2018) Generalized stockwell transform and SVD-based epileptic seizure detection in EEG using random forest. Biocybernetics and Biomedical Engineering 38(3):519–534
Vijayakumar V, Case M, Shirinpour S, He B (2017) Quantifying and characterizing tonic thermal pain across subjects from EEG data using random forest models. IEEE Trans Biomed Eng 64(12):2988–2996
Sai CY, Mokhtar N, Yip HW, Bak LLM, Hasan MS, Arof H, Cumming P, Adenan NAM (2019) Objective identification of pain due to uterine contraction during the first stage of labour using continuous EEG signals and SVM. Sādhanā 44(4):87
Kaur A, Verma K, Bhondekar AP, Shashvat K (2019) Implementation of bagged SVM ensemble model for classification of epileptic states using EEG. Curr Pharm Biotechnol 20(9):755–765
Murugavel AM, Ramakrishnan S (2016) Hierarchical multi-class SVM with ELM kernel for epileptic EEG signal classification. Medical&Biological Engineering&Computing 54(1):149–161
Chang CC, Lin CJ (2011) LIBSVM: a library for support vector machines. ACM transactions on intelligent systems and technology (TIST) 2(3):1–27
Leong WC, Kelani RO, Ahmad Z (2020) Prediction of air pollution index (API) using support vector machine (SVM). Journal of Environmental Chemical Engineering 8(3): 103208
Subasi A (2007) EEG signal classification using wavelet feature extraction and a mixture of expert model. Expert Syst Appl 32(4):1084–1093
Lotte F (2014) A tutorial on EEG signal-processing techniques for mental-state recognition in brain–computer interfaces. Guide to Brain-Computer Music Interfacing, 1st edn. Springer, London, pp 133–161
Subasi A, Ercelebi E (2005) Classification of EEG signals using neural network and logistic regression. Comput Methods Programs Biomed 78(2):87–99
Gleeson K, Zwillich CW, White DP (1990) The influence of increasing ventilator effort on arousal from sleep. Am Rev Respir Dis 142:295–300
Varga AW, Ducca EL, Kishi A, Fischer E, Parekh A, Koushyk V, ... Ayappa I (2016) Effects of aging on slow-wave sleep dynamics and human spatial navigational memory consolidation. Neurobiol Aging 42:142–149
Beninati W, Harris CD, Herold DL, Shepard JW (1999) The effect of snoring and obstructive sleep apnea on the sleep quality of bed partners. Mayo Clin Proc 74:955–958
Chervin RD, Burns JW, Ruzicka DL (2005) Electroencephalographic changes during respiratory cycles predict sleepiness in sleep apnea. Am J Respir Crit Care Med 171:652–658
Author information
Authors and Affiliations
Contributions
MK conceived, designed, analyzed, and supervised the study and participated in writing. MY designed, analyzed, and participated in writing.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This scientific study was approved by the Ethical committee of Ataturk University, Medical School, Erzurum, Turkey, approval No. 06–29/28.05.2020.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kayabekir, M., Yağanoğlu, M. The relationship between snoring sounds and EEG signals on polysomnography. Sleep Breath 26, 1219–1226 (2022). https://doi.org/10.1007/s11325-021-02516-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-021-02516-8