Abstract
Purpose
To evaluate the performance of the NoSAS (neck, obesity, snoring, age, sex) score, the STOP-Bang (snoring, tiredness, observed apneas, blood pressure, body mass index, age, neck circumference, gender) questionnaire, and the Epworth sleepiness score (ESS) as a screening tool for obstructive sleep apnea (OSA) severity based on the apnea-hypopnea index (AHI) and the oxygen desaturation index (ODI).
Methods
Data from 235 patients who were monitored by ambulant polysomnography (PSG) were retrospectively analyzed. OSA severity was classified based on the AHI; similar classification categories were made based on the ODI. Discrimination was assessed by the area under the curve (AUC), while predictive parameters were calculated by four-grid contingency tables.
Results
The NoSAS score and the STOP-Bang questionnaire were both equally adequate screening tools for the AHI and the ODI with AUC ranging from 0.695 to 0.767 and 0.684 to 0.767, respectively. Both questionnaires perform better when used as a continuous variable. The ESS did not show adequate discrimination for screening for OSA (AUC ranging from 0.450 to 0.525). Male gender, age, and BMI proved to be the strongest individual predictors in this cohort.
Conclusion
This is the first study to evaluate the predictive performance of three different screening instruments with respect to both the AHI and the ODI. This is important, due to increasing evidence that the ODI may have a higher reproducibility in the clinical setting. The NoSAS score and the STOP-Bang questionnaire proved to be equally adequate to predict OSA severity based on both the AHI and the ODI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder characterized by repetitive partial or complete upper airway obstruction which often results in decreased arterial oxygen saturation and arousal from sleep [1]. OSA severity is commonly classified based on the apnea-hypopnea index (AHI) [2]. OSA has been associated with cardiovascular and metabolic consequences and is also linked with increased overall mortality [3]. Currently, overnight polysomnography (PSG) is the gold standard for diagnosing the presence and severity of OSA. However, its high expense, relative inaccessibility, and time consumption can delay or impede the diagnosis and treatment of patients with OSA, mainly in areas with limited healthcare resources [4]. Additionally, the increasing number of patients suspected of having OSA and the lack of structured patient interviews contribute to the growing number of patients being referred to sleep clinics [5]. Therefore, simple screening instruments for identifying patients at high risk for OSA have become increasingly important.
Several instruments have been developed over the years including the STOP-Bang questionnaire [6, 7] and the NoSAS score [8]. The STOP-Bang questionnaire shows a high sensitivity and negative predictive value, and therefore is a suitable instrument to rule out patients at risk for OSA [9,10,11,12]. However, it has a low to moderate specificity and it is possible that this will yield a high false-positive rate. Low specificity may result in unnecessary referral to sleep clinics for polysomnography [6, 7]. The NoSAS score has been validated in multiple patient cohorts, and opinions concerning superiority over the STOP-Bang questionnaire differ [8, 10, 13,14,15]. The original validation of the NoSAS score by Marti-Soler et al. describes higher specificity and positive predictive values in comparison with the STOP-Bang questionnaire, while maintaining a moderate to high sensitivity and negative predictive value, therefore allowing to rule out clinically significant OSA and simultaneously reducing the number of unnecessary nocturnal recordings as well as the number of missed diagnosis [8]. The Epworth sleepiness scale (ESS), which was originally designed to assess the extent of daytime sleepiness, has also been suggested as a screening tool for identifying patients at high risk for OSA [16]. However, multiple authors have found the ESS to be inferior to other screening tools for identifying patients at high risk for OSA [11, 12, 17, 18].
The present study reviewed and analyzed a cohort of 235 patients who underwent PSG, using in each case all three instruments: the STOP-Bang questionnaire [6], the NoSAS score [8], and the ESS [16]. Our main objectives were to evaluate the predictive and discriminative performance of the different screening instruments and compare the diagnostic effectiveness of the different methods. Additionally, we aimed to determine which variables independently were the strongest predictors in this cohort.
Recently, it has been suggested that the AHI is susceptible to variability in the clinical setting and that there is a need for an alternative parameter to indicate OSA severity [3, 19,20,21]. An important disadvantage regarding the AHI is that the morphology and duration of the apneas are not taken into account. Longer, deeper apneas might be more significant than shorter, shallow ones [22]. Significant differences in the severity of OSA have been described between patients with a similar AHI [22]. Nocturnal oxygen desaturations are the result of apneas and are important in the pathogenesis and development of complications of OSA [23]. The arterial oxygen desaturation index (ODI) has therefore been proposed as an alternative for the AHI in grading PSG data and classifying OSA severity [23,24,25,26]. The ODI might be more relevant due to the higher reproducibility in the clinical setting [3, 19,20,21]. Furthermore, there is evidence that the ODI is independently associated with prevalent risk factors like hypertension, whereas the AHI is not [19]. Therefore, in the present study, the discriminatory ability of the screening instruments will be evaluated by criteria based on the AHI as well as on the ODI.
Methods
Study design
Data from 235 patients who were monitored by ambulant PSG were retrospectively analyzed. Patient inclusion criteria were patients aging 18 years of age or older, completed clinical data, and completed STOP-Bang questionnaire and NoSAS score. Patient exclusion criteria were previously diagnosed OSA, use of portable sleep studies or respiratory polygraphy, incomplete clinical data, and technically inadequate PSG. In the outpatient clinic, the following clinical parameters were collected for all patients: gender, age, height, weight, body mass index (BMI), neck circumference (NC), self-reported complaints (snoring, daytime sleepiness, and apnea), and self-reported comorbidities (cardiovascular history, hypertension, pulmonary history). The ESS was completed. The clinical parameters were used to calculate the NoSAS score and the STOP-Bang questionnaire.
Screening instruments (supplementary material)
The STOP-Bang questionnaire consists of four questions used in the STOP questionnaire—snoring, tiredness, observed apneas, and hypertension—plus four demographic queries—BMI > 35 kg/m2, age > 50 years old, neck circumference > 40 cm, and male gender. For each question, answering ‘yes’ scores 1, a ‘no’ scores 0. With a total range of 0–8, a total score of ≥ 3 points is considered as a high probability for OSA [6]. The NoSAS score is a 5-item questionnaire which includes neck circumference, obesity, snoring, age, and gender. With a range of 0–17, NoSAS scores 4 points for neck circumference ≥ 40 cm, 3 points for BMI 25–30 kg/m2, 5 points for BMI ≥ 30 kg/m2, 2 points for snoring, 4 points for age > 55 years old, and 2 points for male gender. The total score of ≥ 8 points is considered as a high probability for OSA [8]. The ESS consists of 8 situations, allowing the patients to assess their degree of dozing off or falling asleep in a particular scene during the day, 0 for no dozing, and 1, 2, and 3 for slight, moderate, and high chance of dozing. A total score of ≥ 10 points is considered as excessive daytime sleepiness [16].
Sleep study, scoring, and diagnosis
All patients underwent a full-night PSG at home. PSG included electroencephalography, electrooculography, surface electromyography, nasal airflow and air temperature, thoracoabdominal movements, pulse oximetry, body position, and snoring sounds. Breathing was recorded with nasal pressure and temperature sensors. Scoring of the electronic raw data was performed manually, following the recommendations of the American Academy of Sleep Medicine [2]. Apnea was defined as a decrease of at least 90% of airflow from baseline for > 10 s. Hypopnea was defined as a decrease of at least 30% of airflow from baseline for > 10 s, associated with either an arousal or ≥ 3% arterial oxygen saturation decrease. The mean number of apneas and hypopneas per hour of sleep (AHI) was calculated. The ODI was defined as the mean number of arterial oxygen desaturations ≥ 3% per hour. The severity of OSA was categorized both according to the AHI and to the ODI. By using the AHI, patients were classified as mild (5 ≤ AHI < 15 events/h), moderate (15 ≤ AHI < 30 events/h), or severe (AHI ≥ 30 events/h) according to the 2012 American Academy of Sleep Medicine criteria [2]. For classification according to the ODI, patients were divided into similar groups: mild (5 ≤ ODI < 15 events/h), moderate (15 ≤ ODI < 30 events/h), and severe (ODI ≥ 30 events/h) [27]. Other PSG parameters collected included the apnea index (AI), the AHI in supine position, the AHI in non-supine position, minimal arterial oxygen saturation (minimal SpO2), baseline arterial oxygen saturation (baseline SpO2), average arterial oxygen saturation (average SpO2), and percentage of sleep time with arterial oxygen saturation time below 90% (SpO2 time < 90%).
Statistical analysis
The statistical analysis was performed by using Statistical Package for Social Studies (IBM SPSS Statistics version 24 for Windows, New York, NY, USA). Continuous data are presented as means with standard deviations. Categorical variables are presented as frequencies with percentages. Comparisons between groups were performed using Chi-square tests for categorical variables, unpaired Student’s t test, and univariate analysis of variance (ANOVA) for continuous variables. Discrimination, the ability of a screening tool to distinguish between patients with and without different outcomes, was estimated from the area under the curve (AUC) obtained by receiver operator characteristic (ROC) curves, which may range from 0.5 (no discrimination) to 1.0 (perfect discrimination) [28]. The AUCs were compared using the algorithm previously described by Hanley et al. [29]. Additionally, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for different AHI and ODI cutoffs using four-grid contingency tables, all estimates are reported with their respective 95% confidence interval (CI). The association between various individual demographic and clinical variables and the presence and degree of OSA was established by using a multivariate logistic regression model (backward stepwise selection, p < 0.05). A two-tailed p value < 0.05 was considered statistically significant.
Results
Baseline characteristics
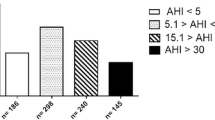
A total of 201 patients met our inclusion criteria; baseline characteristics are mentioned in Table 1. A total of 148 (73.6%) patients were male, aged 50.0 ± 12.6 years, with a mean BMI of 28.0 ± 4.8 kg/m2. Based on the AHI, OSA was present in 159 (79.1%) of the patients; 66 (41.5%) with mild OSA, 45 (28.3%) with moderate OSA, and 48 (30.2%) with severe OSA. Male gender, age, BMI, neck circumference, cardiovascular history, hypertension, snoring, and apneas were all significantly higher in the OSA groups than in the no OSA group. A post hoc Bonferroni test showed a statistically significant difference between no OSA and moderate/severe OSA for male gender (p = 0.008; p = 0.001), age (p = 0.002; p = 0.013), and BMI (p = 0.045; p < 0.001). BMI was also significantly different between mild/moderate OSA and severe OSA (p < 0.001; p = 0.030). Neck circumference (p = 0.043; p = 0.032), cardiovascular history (p = 0.006; p = 0.040), and hypertension (p = 0.004; p = 0.002) all showed a statistically significant difference between no/mild OSA and severe OSA. The ESS did not differ significantly between OSA groups (p = 0.667; p = 0.616). A total of 54.5%, 75.6%, and 85.4% of the patients in the mild, moderate, and severe OSA group, respectively, were classified as high risk of OSA according to the NoSAS score (cutoff ≥ 8; p < 0.001). A total of 97%, 100%, and 100% in the mild, moderate, and severe OSA group, respectively, were classified as high risk of OSA according to the STOP-Bang questionnaire (cutoff ≥ 3; p < 0.001). Polysomnography results (AHI, ODI ≥ 3%, minimal SpO2, average SpO2, and SpO2 time < 90%) were all significantly different between the OSA and no OSA groups (p < 0.001; p < 0.001; p < 0.001; p < 0.001; p = 0.001). Notable is the percentage of patients with positional sleep apnea which was also statistically significant between the groups (p < 0.001). A post hoc Bonferroni test shows that the difference was significant between no OSA and all OSA severity groups (p < 0.001) and mild OSA and severe OSA (p = 0.05).
Performance of instruments
The predictive performance of the different screening instruments as categorical variable is shown in Table 2. For screening on different cut-off points of AHI and ODI severity, the sensitivity of the NoSAS score varies from 0.70 to 0.92 (AHI > 5 and AHI > 15, respectively). The specificity varies from 0.37 to 0.55 (AHI > 15 and AHI > 5, respectively). The STOP-Bang questionnaire showed the highest sensitivity varying from 0.99 to 1.00. However, the specificity was lower varying from 0.06 to 0.17. The highest specificity was obtained by the ESS, varying from 0.79 to 0.83, with a low sensitivity varying from 0.15 to 0.19. Figure 1 shows the ROC curves and the corresponding AUC of the three screening instruments on different levels of AHI and ODI severity. The screening instruments are presented as continuous variables. The ESS did not show adequate discrimination for screening for AHI and ODI with an AUC ranging from 0.450 to 0.525. The NoSAS score and the STOP-Bang questionnaire were both equally adequate screening tools for the AHI and the ODI with AUC ranging from 0.695 to 0.767 and 0.684 to 0.767, respectively (all comparisons with p value > 0.05). The discriminatory ability of the NoSAS score and the STOP-Bang questionnaire was similar in relation to both the AHI and the ODI (all comparisons with p value > 0.05). When used as categorical variable, the AUC of the NoSAS score ranged from 0.620 to 0.684 (cutoff ≥ 8), the AUC of the STOP-Bang questionnaire ranged from 0.529 to 0.577 (cutoff ≥ 3) (Table 2). Both instruments performed better when used as continuous variable than as categorical variable. However, only for the STOP-Bang questionnaire, this difference proved to be significant (all comparisons except AHI ≥ 5 with p value < 0.05).
Discriminatory ability reported as area under the curve (AUC) (95% CI). The NoSAS score, the STOP-Bang questionnaire, and the ESS are presented as continuous variables. OSA severity is classified based on AHI ≥ 5 (any OSA), AHI ≥ 15 (moderate to severe OSA), and AHI ≥ 30 (severe OSA). The ODI ≥ 3% is subdivided into ODI ≥ 5, ODI ≥ 15, and ODI ≥ 30. The NoSAS score performed similar when compared with the STOP-Bang questionnaire on all cutoff points (all comparisons with p value > 0.05). The ESS presented lower discrimination than presented by the NoSAS score and the STOP-Bang questionnaire on all cutoff points (all comparisons with p value < 0.05)
Predicting OSA
Multivariate logistic regression analyses were performed in order to establish the association between various individual demographic and clinical variables and the presence and degree of OSA categorized by the AHI and the ODI. Gender, age, and BMI proved to be the strongest predictors for any OSA (AHI ≥ 5) (p < 0.001; p < 0.001; p = 0.004), moderate to severe OSA (AHI ≥ 15) (p < 0.001; p < 0.001; p < 0.001), ODI ≥ 5 (p = 0.001; p = 0.001; p = 0.001), and ODI ≥ 15 (p < 0.001; p < 0.001; p < 0.001). Gender, BMI, and self-reported history of hypertension proved to be or the strongest predictors for severe OSA (AHI ≥ 30) (p = 0.028; p < 0.001; p = 0.028) and ODI ≥ 30 (p = 0.024; p < 0.001; p = 0.034). The ROC curves of the estimated predictive probability, the NoSAS score, and the STOP-Bang questionnaire with cutoff points AHI ≥ 15 and ODI ≥ 15 are shown in Fig. 2. The AUC of the estimated predicted probability was 0.784 when differentiating for AHI ≥ 15 and 0.805 when differentiating for ODI ≥ 15. The predicted probability performs similar to the NoSAS score and the STOP-Bang questionnaire (all comparisons with p value > 0.05).
Discriminatory ability reported as area under the curve (AUC) (95% CI). The NoSAS score and the STOP-Bang questionnaire are presented as continuous variables. The green ROC curve shows the plotted predicted probability of gender, age, and BMI. The predicted probability performs similar to the NoSAS score and the STOP-Bang questionnaire (all comparisons with p value > 0.05). The ROC curves are presented at AHI ≥ 15 and ODI ≥ 15
Discussion
The present study shows that both the NoSAS score and the STOP-Bang questionnaire, but not the ESS, were equally useful to detect patients at high risk for OSA. In this cohort, the STOP-Bang questionnaire had the highest sensitivity, with a low specificity. The NoSAS score had a higher specificity and PPV, while maintaining a moderate to high sensitivity. The ESS had the highest specificity, with a low sensitivity. This is in correspondence with what was found by previous authors [8, 10, 11, 13, 18, 30]. The discriminatory ability of the NoSAS score and the STOP-Bang questionnaire was similar in relation to both the AHI and the ODI. However, due to the low specificity and positive predictive value of the STOP-Bang questionnaire, it is possible that the STOP-Bang will yield a large proportion of false-positive cases if used in a wrong patient group and therefore increase the number of unnecessary nocturnal recordings, whereas the NoSAS score describes higher specificity and positive predictive values, while maintaining a moderate to high sensitivity and negative predictive value.
The discriminatory ability of the NoSAS score and the STOP-Bang questionnaire as a categorical variable was compared with the discriminatory ability as a continuous variable. As expected, the discriminatory ability is higher when the instrument is used as a continuous variable. However, only for the STOP-Bang questionnaire, this difference proved to be significant. Previous studies have already suggested that the probability of moderate to severe OSA increases in direct proportion to the STOP-Bang score, and therefore, the questionnaire should be used as a continuous rather than as a categorical variable. Chung et al. suggested patients with a STOP-Bang score of 0 to 2 to be classified as being at low risk for moderate to severe OSA. Those with a STOP-Bang score of 5 to 8 can be classified as being at high risk for moderate to severe OSA. In patients with a STOP-Bang score of 3 or 4, specific combinations of positive items should be examined further to ensure proper classification [6]. The NoSAS score has previously been presented as categorical variable with various cutoff points [8, 10, 13, 14, 30]. However, according to our study results, a similar scoring system to the STOP-Bang questionnaire can be considered. Coutinho Costa et al. suggested a similar approach, prioritizing patients depending on their score. Patients with a score of 0–5 are to be classified as low probability of OSA—particularly moderate to severe OSA; a score ≥ 7 are to be classified as probable OSA; a score ≥ 12 as a high probability of OSA—particularly moderate to severe OSA [14].
In the present cohort, male gender, age, and BMI showed to be the strongest individual predictors for OSA severity based on the AHI and the ODI. The discriminatory ability of the three variables combined was similar to the discriminatory ability of the NoSAS score and the STOP-Bang questionnaire. In future, this might present interesting opportunities to design a screening tool based on only three variables. As an alternative, the weighing factor of the variables gender, age, and BMI could be set higher in the existing screening instruments. A similar approach was suggested by Chung et al. for the STOP-Bang questionnaire, introducing male gender, BMI, and neck circumference as high-risk variables [6].
Clinical implications
This is the first study that evaluated the predictive performance of three different screening instruments with respect to both the AHI and the ODI. This is relevant, due to increasing evidence that the ODI has a higher reproducibility in the clinical setting [19,20,21]. Furthermore, significant differences in the severity of OSA have been described between patients with a similar AHI. Presumably, this is due to the fact that the morphology and duration of the apneas are not taken into account in the AHI [22]. In the present study, the NoSAS and STOP-Bang screening instruments both have a high discriminatory ability to predict OSA severity based on the AHI and the ODI. The ESS, however, was not able to detect patients at high risk for OSA and should, therefore, not be used as a screening instrument.
Limitations and strengths
In general, the use of a retrospective analysis to validate the predictive value of different screening instruments is less ideal than a prospective study. In this observational study, however, our center had collected data prior to PSG monitoring, thus maintaining a high credibility for this retrospective study. Most patients were referred to the sleep clinic because they were suspected of having sleep-related problems. Therefore, it is possible that a selection bias was introduced, since the questionnaire was applied only to the suspected individuals. The great prevalence of OSA in this study population could affect the interpretation of the screening instruments. Contrarily, the present study has several important strengths: this is the first study that has evaluated the predictive value of different screening instruments on the ODI. As the ODI is gaining attention as new variable to classify OSA severity, this is an important new insight. Furthermore, all patients were evaluated with a full PSG and scored according to the current guidelines proposed by the American Academy of Sleep Medicine in 2012 [2].
Data availability
The dataset is available on request from Christianne Veugen, Department of Otorhinolaryngology Head and Neck Surgery, Sint Antonius Hospital, Koekoekslaan 1, 3435 CM Nieuwegein, the Netherlands. E: c.veugen@antoniusziekenhuis.nl.
Abbreviations
- AHI:
-
apnea-hypopnea index
- AI:
-
apnea index
- ANOVA:
-
analysis of variance
- AUC:
-
area under the curve
- BMI:
-
body mass index
- CI:
-
confidence interval
- ESS:
-
Epworth Sleepiness Scale
- NC:
-
neck circumference
- NPV:
-
negative predictive value
- ODI:
-
oxygen desaturation index
- OSA:
-
obstructive sleep apnea
- PPV:
-
positive predictive value
- PSG:
-
polysomnography
- ROC:
-
receiver operating characteristic
References
American Academy of Sleep Medicine Task Force (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22:667–689
Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, Troester MT, Vaughn BV (2017) AASM scoring manual updates for 2017 (version 2.4). J Clin Sleep Med 13:665–666. https://doi.org/10.5664/jcsm.6576
Muraja-Murro A, Kulkas A, Hiltunen M, Kupari S, Hukkanen T, Tiihonen P, Mervaala E, Töyräs J (2013) The severity of individual obstruction events is related to increased mortality rate in severe obstructive sleep apnea. J Sleep Res 22:663–669. https://doi.org/10.1111/jsr.12070
Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J (2004) Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med 169:668–672. https://doi.org/10.1164/rccm.200308-1124pp
Reuveni H, Tarasiuk A, Wainstock T, Ziv A, Elhayany A, Tal A (2004) Awareness level of obstructive sleep apnea syndrome during routine unstructured interviews of a standardized patient by primary care physicians. Sleep 27:1518–1524. https://doi.org/10.1093/sleep/27.8.1518
Chung F, Abdullah HR, Liao P (2016) STOP-bang questionnaire a practical approach to screen for obstructive sleep apnea. Chest 149:631–638. https://doi.org/10.1378/chest.15-0903
Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM (2008) STOP Questionnaire. Anesthesiology 108:812–821. https://doi.org/10.1097/aln.0b013e31816d83e4
Marti-Soler H, Hirotsu C, Marques-Vidal P, Vollenweider P, Waeber G, Preisig M, Tafti M, Tufik SB, Bittencourt L, Tufik S, Haba-Rubio J, Heinzer R (2016) The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med 4:742–748. https://doi.org/10.1016/S2213-2600(16)30075-3
Rebelo-Marques A, Vicente C, Valentim B, Agostinho M, Pereira R, Teixeira MF, Moita J (2018) STOP-Bang questionnaire: the validation of a Portuguese version as a screening tool for obstructive sleep apnea (OSA) in primary care. Sleep Breath 22:757–765. https://doi.org/10.1007/s11325-017-1608-0
Hong C, Chen R, Qing S, Kuang A, Yang HJ, Su X, Zhao D, Wu K, Zhang N (2018) Validation of the NoSAS score for the screening of sleep-disordered breathing: a hospital-based retrospective study in China. J Clin Sleep Med 14:191–197. https://doi.org/10.5664/jcsm.6930
Duarte RLM, Mello FCQ, Magalhães-da-Silveira FJ, Oliveira-e-Sá TS, Rabahi MF, Gozal D (2019) Comparative performance of screening instruments for obstructive sleep apnea in morbidly obese patients referred to a sleep laboratory: a prospective cross-sectional study. Sleep Breath 23:1123–1132. https://doi.org/10.1007/s11325-019-01791-w
Silva GE, Vana KD, Goodwin JL, Sherrill DL, Quan SF (2011) Identification of patients with sleep disordered breathing: comparing the four-variable screening tool, STOP, STOP-Bang, and Epworth sleepiness scales. J Clin Sleep Med 7:467–472. https://doi.org/10.5664/JCSM.1308
Tan A, Hong Y, Tan LWL, van Dam RM, Cheung YY, Lee CH (2017) Validation of NoSAS score for screening of sleep-disordered breathing in a multiethnic Asian population. Sleep Breath 21:1033–1038. https://doi.org/10.1007/s11325-016-1455-4
Coutinho Costa J, Rebelo-Marques A, Machado JN, Gama JMR, Santos C, Teixeira F, Moita J (2019) Validation of NoSAS (neck, obesity, snoring, age, sex) score as a screening tool for obstructive sleep apnea: analysis in a sleep clinic. Pulmonology 25:263–270. https://doi.org/10.1016/j.pulmoe.2019.04.004
Giampá SQC, Pedrosa RP, Gonzaga CC, Bertolami A, Amodeo C, Furlan SF, Bortolotto LA, Lorenzi-Filho G, Drager LF (2018) Performance of NoSAS score versus Berlin questionnaire for screening obstructive sleep apnoea in patients with resistant hypertension. J Hum Hypertens 32:518–523. https://doi.org/10.1038/s41371-018-0072-z
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545. https://doi.org/10.1093/sleep/14.6.540
Chiu HY, Chen PY, Chuang LP, Chen NH, Tu YK, Hsieh YJ, Wang YC, Guilleminault C (2017) Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev 36:57–70. https://doi.org/10.1016/j.smrv.2016.10.004
Duarte RLM, Magalhães-da-Silveira FJ, Oliveira-e-Sá TS, Rabahi MF, Mello FCQ, Gozal D (2019) Predicting obstructive sleep apnea in patients with insomnia: a comparative study with four screening instruments. Lung 197:451–458. https://doi.org/10.1007/s00408-019-00232-5
Tkacova R, McNicholas WT, Javorsky M, Fietze I, Sliwinski P, Parati G, Grote L, Hedner J (2014) Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea Database cohort study. Eur Respir J 44:931–941. https://doi.org/10.1183/09031936.00225113
Vos P. Richtlijn obstructief slaapapneu (OSA) bij volwassenen
Nieto FJ, Young TB, Lind BK (2000) Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 283:1829–1836. https://doi.org/10.1001/jama.283.14.1829
Kulkas A, Tiihonen P, Julkunen P, Mervaala E, Töyräs J (2013) Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index. Med Biol Eng Comput 51:697–708. https://doi.org/10.1007/s11517-013-1039-4
Temirbekoy D, Gunes S, Yazici ZM, Sayin İ (2018) The ignored parameter in the diagnosis of obstructive sleep apnea syndrome the oxygen desaturation index. Turk Otolarengoloji Arsivi/Turkish Arch Otolaryngol:1–6. https://doi.org/10.5152/tao.2018.3025
Fietze I, Dingli K, Diefenbach K, Douglas NJ, Glos M, Tallafuss M, Terhalle W, Witt C (2004) Night-to-night variation of the oxygen desaturation index in sleep apnoea syndrome. Eur Respir J 24:987–993. https://doi.org/10.1183/09031936.04.00100203
Tsai CM, Kang CH, Su MC, Lin HC, Huang EY, Chen CC, Hung JC, Niu CK, Liao DL, Yu HR (2013) Usefulness of desaturation index for the assessment of obstructive sleep apnea syndrome in children. Int J Pediatr Otorhinolaryngol 77:1286–1290. https://doi.org/10.1016/j.ijporl.2013.05.011
Levendowski DJ, Hamilton GS, St. Louis EK, Penzel T, Dawson D, Westbrook PR (2019) A comparison between auto-scored apnea-hypopnea index and oxygen desaturation index in the characterization of positional obstructive sleep apnea. Nat Sci Sleep 11:69–78. https://doi.org/10.2147/NSS.S204830
Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y (2012) Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg 114:993–1000. https://doi.org/10.1213/ANE.0b013e318248f4f5
Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW (2010) Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 21:128–138. https://doi.org/10.1097/EDE.0b013e3181c30fb2
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36. https://doi.org/10.1148/radiology.143.1.7063747
Guichard K, Marti-Soler H, Micoulaud-Franchi JA, Philip P, Marques-Vidal P, Vollenweider P, Waeber G, Preisig M, Haba-Rubio J, Heinzer R (2018) The NoSAS score: a new and simple screening tool for obstructive sleep apnea syndrome in depressive disorder. J Affect Disord 227:136–140. https://doi.org/10.1016/j.jad.2017.10.015
Funding
There was no funding received for this research.
Author information
Authors and Affiliations
Contributions
Data collection was performed by ET. Data analysis was performed by LO and CV. CV wrote the manuscript. MK, RS, and MC provided scientific oversight. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Data on study subjects was collected and stored anonymously to protect personal information. This manuscript does not report on a clinical trial, and therefore was not registered in a clinical trial registration.
Manuscript approval
All authors declare that they have seen and approved the final version of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 12 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Veugen, C.C.A.F.M., Teunissen, E.M., den Otter, L.A.S. et al. Prediction of obstructive sleep apnea: comparative performance of three screening instruments on the apnea-hypopnea index and the oxygen desaturation index. Sleep Breath 25, 1267–1275 (2021). https://doi.org/10.1007/s11325-020-02219-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-020-02219-6