Abstract
Objective
Airway inflammation plays an important role in obstructive sleep apnea (OSA); exhaled nitric oxide is regarded as a noninvasive marker of airway inflammation. The aim of this study was to evaluate fractional exhaled nitric oxide (FeNO) and nasal nitric oxide (nNO) in patients with OSA.
Methods
Seventy-five patients with OSA and 30 health controls were enrolled in this study. FeNO and nNO were measured before and after sleep. Nasal lavage was performed in 31 non-smoking individuals immediately after NO measurement in the morning. The sample of nasal lavage was taken for cell classification and analyzing interleukin 6 (IL-6) and interleukin 8 (IL-8).
Results
Both FeNO and nNO were significantly higher in OSA (before sleep FeNO 21.08 ± 8.79 ppb vs.16.90 ± 6.86 ppb, p = 0.022; after sleep FeNO 25.57 ± 15.58 ppb vs.18.07 ± 6.25 ppb, p = 0.003; before sleep nNO 487.03 ± 115.83 ppb vs. 413.37 ± 73.10 ppb, p = 0.001; after sleep nNO 550.07 ± 130.24 ppb vs. 460.43 ± 109.77 ppb, p < 0.001). Furthermore, in non-smoking OSA, nNO levels were positively correlated with apnea hypopnea index (AHI) and average decrease of pulse arterial oxygen saturation (SpO2); after sleep, nNO was also positively associated to recording time with SpO2 < 90% and negatively associated to minimum SpO2. Both before and after sleep nNO levels were positively correlated with the percentage of neutrophils in nasal lavage (r = 0.528, p = 0.014; r = 0.702, p < 0.001, respectively). Additionally, before sleep nNO was also positively associated with IL-6 (r = 0.586, p = 0.005) and IL-8 (r = 0.520, p = 0.016) concentration.
Conclusion
This study sustains the presence of airway inflammation in OSA patients with the increase of FeNO and nNO. The data suggests nNO might have greater value than FeNO since it positively correlated with OSA severity, and nNO is a potential bio-marker of nasal inflammation in non-smoking OSA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most important characteristic of obstructive sleep apnea (OSA) is repeated pharyngeal collapses during sleep. Local inflammation at nose, pharyngeal, and laryngeal aggravates upper airway narrowing and increases the risk of OSA. Otherwise, the mechanical damage of recurrent upper airway closure and the oxidative stress caused by intermittent oxygen desaturation induce local and systemic inflammation in OSA patients. Non-invasive methods such as exhaled gases, exhaled breath condensate, and induced sputum can be used to detect these inflammation and oxidative stress in patients with OSA [1].
Exhaled nitric oxide is regarded as a non-invasive marker of airway inflammation [2]. Several studies have evaluated fractional exhaled nitric oxide (FeNO) in OSA, but they got controversial results. In our mate-analysis, we found a high FeNO in OSA [3], but its level was still very close to the upper limit value of the health (25 ppb) which makes it impractical to be used in clinical setting. Previous study revealed a positive correlation between FeNO and nNO levels in patients with allergic rhinitis and health volunteers [4]. As nasal nitric oxide (nNO) is several times higher than FeNO [5], we hypothesize the difference of nNO level in OSA patients, and the health adults would also be much greater. Unfortunately, only six researches about nNO in patients with OSA were published, and among them, three were in pediatric OSA [6,7,8]; the other three were in adults with different methods of measuring nNO [9,10,11]. The levels of nNO in patients with OSA varied widely (from 28.4 to 610.03 ppb) in different studies, and all of these studies included less than 50 OSA patients. In the American Thoracic Society/European Respiratory Society (ATS/ERS) recommendations for standardized procedures for the measurement of nNO, the importance of velum closure was emphasized [12], to prevent loss of NO via the posterior velopharyngeal aperture or enter of lower respiratory air.
Therefore, this study had two objectives. First, we evaluated the levels of nNO in patients with OSA and healthy subjects by the ATS/ERS recommended method. Second, we investigated the relationship between nNO and FeNO and inflammatory factors in nasal lavage, in order to know whether nNO might be a noninvasive marker of upper airway inflammation in patients with OSA.
Methods
Study subjects
Seventy-five patients consecutively admitted to sleep center of Peking Union Medical College Hospital (PUMCH) were included. All patients were newly diagnosed OSA. Health controls were recruited from Medical Examination Center. We excluded subjects with respiratory disease including asthma, chronic obstructive pulmonary disease, infection in the last 4 weeks, rhinitis, atopic individuals, organic failure, auto-immunity diseases, treatment with oral or inhalation corticosteroids, and drugs including arginine. This study was approved by ethics committee of PUMCH, and informed consents were obtained from all participants.
Study design
All participants admitted to sleep center of PUMCH at 8 p.m. Medical history and Epworth Sleepiness Scale (ESS) questionnaires were asked, and personal parameters were measured by well-trained researchers. Lung ventilation function including forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were assessed by spirometry (SuperSpiro, Micro Medical, UK).
Exhaled nitric oxide measurement
FeNO and nNO were measured before and after sleep (9:30 to 10:00 p.m. and 6:00 to 6:30 a.m.) by NIOX MINO® (Aerocrine, Sweden). All measures were performed based on the ATS/ERS guidelines and the instruction of NIOX MINO® [13]. When FeNO was measured, subjects kept expiring at a constant flow of 50 mL/s for at least 10 s in a sitting position. When nNO was measured, subjects kept expiring with the micro-air pressure gauge ranging between 10 and 20 cmH2O until NIOX MINO® draw enough air from the nose (Supplement Fig. 1). Heavy exercise and smoking were inhibited 1 h before the test.
Polysomnography
Every subject performed standard overnight polysomnography (PSG, Embla N7000, Natus Medical Incorporated, USA) from 10 pm. to 6 am. in the sleep laboratory. Electroencephalography, electrooculography, and chin electromyography recordings were recorded with surface electrodes according to standard methods. Airflow was monitored by nasal pressure. Thoracic and abdominal movements were assessed by respiratory inductive plethysmography which was recorded by belts connected to transducers. All the night recordings of pulse oxygen saturation (SpO2) was continuously acquired by an oximeter being attached to a finger. Electrocardiography and sleep positions also were noted. The sleep records were scored based on standardized criteria recommended by the American Academy of Sleep Medicine [14]. The sleep records were auto-scored by software, and then manually scored by a sleep laboratory expert. OSA was defined by an apnea hypopnea index (AHI) of five or more episodes per hour, associated with daytime somnolence.
Nasal lavage
After repeatedly measured FeNO and nNO in the morning, 21 patients with OSA and ten matched health controls collected nasal lavage by an atomized nasal washing machine (Neb-nid, Flaem Nuova SPA, Italy). Rhino-Clear was filled with 6 mL natural saline and washed one nasal cavity. The other one was also washed in the same way. The collected nasal lavage samples were blindly examined within 2 h. It was filtered through gauze to remove mucus, and then centrifuged at 3000 rpm for 5 min. The supernatant was aspired for analyzing interleukin 6 (IL-6) and interleukin 8 (IL-8). The cell pellet was resuspended in 0.5 ml natural saline. The cell suspension was placed in a cytocentrifuge (Thermo Scientific Cytospin 4, Shandon, UK), and cytospin preparations were made at 800 rpm for 4 min. Cytospin slides were fixed with methanol and were stained with Wright-Giemsa by an automatic slides staining machine (Sysmex, Japan). Two investigators independently counted at least 200 cells per slide to classify cells under light microscopy and with the assistance of an electrical hemocytometer (BCC-B, Goodline Medical Technology Co. Ltd. China). IL-6 and IL-8 were measured by IMMULITE 1000 Automatic Chemiluminescence Immunoassay (Siemens Medical, USA). The intra-assay coefficients of variation (CV) were 3.5–6.2% and 3.6–3.8%, and the inter-assay CV were 5.1–7.5% and 5.2–7.4%, respectively. Both of their sensitivity were 2 pg/ml.
Statistical analysis
Data analyses were performed using SPSS software version 21.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 6.02 (GraphPad Software Inc., La Jolla, USA). Continuous variables were tested for normal distribution by Kolmogorov–Smirnov test. Results were expressed as mean ± standard deviations and as the median with interquartile range. Comparisons were made by Student’s t test or Mann–Whitney U test or one-way ANOVA being followed by a post hoc Tukey test for quantitative variables and chi-square test or Kruskal–Wallis nonparametric test for qualitative variables, as appropriate. Exhaled NO levels before and after PSG were compared using paired t test. Linear correlations between exhaled NO levels and clinical characteristics or other parameters were done using Pearson’s or Spearman’s method. All reported p values are two-sided, and p < 0.05 was considered to be statistically significant.
Results
Characteristics of study subjects
The characteristics of the participants are presented in Table 1. The OSA group was consisted of 75 male patients and was middle-aged (40.39 ± 9.58 years) and overweight with the mean body mass index (BMI) 27.73 kg/m2. The control group contained 30 healthy middle-aged males who were also a little bit overweight (mean BMI 24.41 kg/m2). All individuals had normal lung function. The median AHI was 28.1/h for OSA patients and 0.85/h for controls.
Exhaled NO
As shown in Fig. 1, both before sleep and after sleep FeNO in patients with OSA were significantly higher than values in the healthy (21.08 ± 8.78 vs. 16.90 ± 6.86 ppb, p = 0.022, 25.57 ± 15.57 vs. 18.07 ± 6.25 ppb, p = 0.003, respectively). Similarly, nNO level was also higher in OSA group (before sleep nNO 487.03 ± 115.83 vs. 413.37 ± 73.10 ppb, p < 0.001, and after sleep nNO 550.07 ± 130.24 vs. 460.43 ± 109.77 ppb, p = 0.001, respectively). Interestingly, we found both FeNO and nNO levels significantly increased after sleep compared with their values before sleep (p < 0.001) in the OSA group, while only nNO levels increased after sleep in health controls (p = 0.034).
Then, we divided the OSA group into different subgroups according to BMI, AHI, hypertension, and smoking, to compare FeNO and nNO in different groups. We found in the no-smoking OSA group, all of before and sleep FeNO and nNO significantly higher than that in no-smoking health controls (Table 2), while these values were similar between smoking patients with OSA and the healthy.
Cell classification and IL-6, IL-8 in nasal lavage
The characteristics of nonsmokers (21 with OSA and 10 controls) were presented in supplement Table 1. We found the percentage of neutrophils, lymphocytes, and eosinophils were significantly higher in OSA patients compared with the healthy subjects (Table 3). Measurements of IL-6 and IL-8 also showed higher values in OSA patients compared with control subjects (Table 3).
Correlation between after sleep exhaled NO and sleep apnea parameters and nasal lavage inflammation markers
In the OSA non-smokers, significant correlations were found between before sleep nNO, after sleep nNO, and AHI (r = 0.387, p = 0.003; r = 0.301, p = 0.023, respectively). Minimum SpO2 and total sleep time with SpO2 < 90% (TST SpO2 < 90%) were also correlated with after sleep nNO. While, the levels of FeNO before and after sleep were not significantly correlated with PSG parameters. There were direct correlation between the percentage of neutrophils and nNO (before sleep nNO r = 0.528, p = 0.014; after sleep nNO r = 0.702, p < 0.001, Fig. 2). Before sleep nNO were also positively correlated with the concentration of IL-6 (r = 0.582, p = 0.005) and IL-8 (r = 0.520, p = 0.016) in nasal lavage of non-smoking OSA patients. Additionally, all of neutrophils, the concentration of IL-6 and IL-8 positively associated with AHI (r = 0.672, p = 0.001; r = 0.676, p = 0.001; r = 0.732, p < 0.001, respectively).
After sleep nasal nitric oxide for detecting non-smoking patients of OSA
Because the levels of after sleep nNO were highly correlated to the severity of OSA as measured by AHI and SpO2, we try to used it for screening nonsmoking OSA. The area under receiver operating characteristic (ROC) curve was statistically significant (p < 0.001; AUC 0.731 95% CI [0.613–0.848]) (Fig. 3). The highest Youden index was at nNO level of 442 ppb with sensitivity of 84.2% and specificity of 55.6%. Another threshold of nNO at 626.5 ppb yielded higher specificity of 96.3% but lower sensitivity of 22.8%.
Discussion
This study found that both FeNO and nNO levels were significantly higher in OSA patients than in controls. Moreover, FeNO and nNO increased after sleep in OSA patients, while nNO also slightly increased after sleep in healthy group. In smoker with OSA, the level of FeNO and nNO were lower than non-smoking OSA patients. The levels of nNO before and after sleep were closely related with sleep apnea severity as expressed by AHI, and after sleep nNO was also correlated with oxygen desaturation in non-smoking OSA. We demonstrated a neutrophilic nasal inflammation in OSA patients even when they had not shown symptoms associated with rhinitis such as runny nose or sneezing. The level of nNO was associated with nasal inflammatory marker including percentage of neutrophils, released IL-6, and IL-8.
Several studies have been published about the FeNO variation in OSA patients, but their results are controversial. The result of our study was very close to these in published meta-analysis [3] as after sleep FeNO was higher than controls by 6.50 ppb and after sleep FeNO was also increased 4.49 ppb compared with before sleep FeNO in OSA group. In non-smoking OSA patients of our study, neither before sleep FeNO, after sleep FeNO, nor ΔFeNO related to PSG parameters, which indicate FeNO, could not reflect the severity of OSA. Devouassoux et al. [15] and Depalo et al. [16] demonstrated a high neutrophil percentage and IL-8 concentration in induced sputum of untreated OSA. Depalo [16] further detected higher iNOS expression in neutrophils which was positive correlated with FeNO. These two studies shown the high FeNO come from bronchial neutrophils in OSA patients. In their studies, FeNO positively correlated with AHI, but we did not find such correlation. In the meta-analysis [3] included 688 OSA patients and 366 health controls, FeNO also did not relate to AHI. Even though OSA patients have upper airway inflammation and with a higher FeNO level, FeNO still cannot be a marker of OSA because it did not relate with OSA severity.
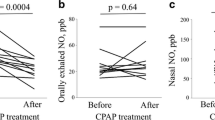
A major source of nNO was paranasal sinuses [17, 18] in healthy individuals. Nasal NO have important local as well as distal effects in keeping sinuses sterile, stimulating ciliary motility, and regulating pulmonary function by improving oxygen uptake and reducing pulmonary vascular resistance. Nasal NO has been used as a reliable test for primary ciliary dyskinesia [19] and a surrogate marker of nasal inflammation in allergic rhinitis [12, 20]. Up to now, only three studies on nNO concentration in adult OSA patients have been published [9,10,11], but their results are significantly different. In 1997, Olopade firstly measured nNO by off-line method (collect exhaled nasal gas and then test nNO concentration) [9]. With the development of technology and equipment, off-line method has been replaced by on-line way. Petrosyan [10] found a higher nNO in OSA subjects than obesity controls, and it was rectified after 1-month continuous positive airway pressure (CPAP) therapy. In his research, end-exhaled CO2 was measured simultaneously to make sure a satisfactory breath holding during the measurement of nNO. It got reliable results with mean nNO 610.3 ± 222.5 ppb in OSA patients. This level was a little higher than our results 550.07 ± 130.24 ppb because we used different gauge. LR2000 chemiluminenscence analyzer often get a higher result than NIOX MINO® [21]. In the recently published study of Hamada [11], nNO level was measured and calculated in a different way which lead to a much lower nNO level (from 47.9 to 76.9 ppb) in their study. In our study, we optimized the measurement of nNO by slowly exhaling orally against a resistance (micro-air pressure gauge with visual pressure) to keep velum closed. We found a higher nNO in non-smoking OSA group and the after sleep nNO was further increased than before sleep level. In non-smoking OSA patients, after sleep nNO was positively correlated with AHI and TST SpO2 < 90% and negatively associated to minimum SpO2. So nNO potentially show upper airway inflammation in OSA patients.
Nasal inflammation had been observed in OSA patients for many years [22], and the prevalence of chronic rhinitis or rhinosinusitis in OSA patients was 3.18 times of that in the healthy [23]. The most important mechanisms of upper airway inflammation in OSA was hypoxia-reflexogen-induced inflammatory cytokine release [24]. High level of inflammatory factors upregulate NF-κB pathway and increase iNOS expression further increase NO production [25]. Consistent with the animal studies, nNO aslo positively correlated with AHI, TST SpO2 < 90% and negatively related to minimum SpO2. Higher percentage of neutrophils was observed in nasal lavage of non-smoking OSA patients with no symptom rhinitis, which was similar with the result of other studies [22, 26]. Cellular classification of induced sputum in OSA patients also found a higher percentage of neutrophils [15, 16]. Depalo measured iNOS expression in sputum cells by immunocytochemistry and showed higher iNOS expression in neutrophils of OSA patients. This indicated the origin of increased exhaled NO level. Similarly, the high percentage of neutrophils in nasal lavage of OSA was positively correlated with nNO.
Studies showed that the concentration of IL-6 and IL-8 increased in exhaled breath condensate and induced sputum [27,28,29]. We observed an increase of IL-6 and IL-8 in nasal lavage of non-smoking OSA. All of IL-6, IL-8, and percentage of neutrophils positively correlated with AHI, which indicated they were associated with OSA severity. Researches have shown IL-6 and IL-8 may mediate nasal inflammation in OSA patients. Hypoxia can activate NF-κB pathway and prompt release of IL-6 [30] and IL-8 [31] to evoke inflammation. For the best of our knowledge, our study firstly observed the positive correlation between nNO and percentage of neutrophils, IL-6, IL-8 in nasal lavage, and AHI, which indicates that nNO was a marker of nasal inflammation and severity of OSA. However, in our study, the expression of iNOS in neutrophils was not tested, and the effect of CPAP therapy on nNO was not followed.
As nNO level related to OSA severity, we made a ROC curve to primarily evaluate whether nNO could be used in clinical screening of OSA. We suggest when the after sleep nNO higher than 626 ppb, PSG should be recommended to confirm OSA diagnosis. This cutoff value of nNO has a high specificity but low sensitivity to detect OSA. As there are still no effective questionnaires or models to screen OSA so far, further researches are needed to combine nNO with other methods to improve the accuracy in predicting OSA [28]. However, only few studies with small samples about nNO are available at present, and the normal range of nNO still need to be worked out. So, the cutoff value need further verification in large population. Therefore, a great distance exists from the clinical use of nNO.
Moreover, the percentage of lymph cell and eosinophil in nasal lavage of OSA patients was higher than that of health controls. But their percentages were too small to meet the criterion of allergic rhinitis; we speculate they were also a performance of nasal inflammation. In pharyngeal lavage, lymph especially CD4+ T cells, IL-6, and IL-8 were higher in OSA patients than in snorers or healthy controls, and they were also positively correlated with AHI. All of them decreased significantly after 1 year of therapy with CPAP or surgery [32].
Nasal mucosa swelling and cavity obstruction, being caused by nasal inflammation, can increase the severity of OSA. A lot of OSA patients have subclinical nasal inflammation without common symptoms, so it is very hard to be diagnosed. But this upper airway inflammation may be closely connected with noncompliance with nasal CPAP therapy [26]. So, further studies are still needed to detect the relationship between nNO and nasal inflammation, CPAP complication, and the effect of CPAP, in order to know whether nNO can be a predictor of CPAP complication or follow-up marker of CPAP effects.
We found a lower FeNO and nNO level in smoking OSA than non-smoking OSA patients, inconsistent with the previous studies which also indicate a lower FeNO in smokers [33, 34]. Because smoking can inhibit the expression of iNOS to reduce production and activity of NO [33, 35], and the harmful gases such as free radicals and superoxide in smoking have chemical reaction with NO to form nitrite amine. Smoking and OSA have opposite effects on FeNO and nNO level, so the concentration of FeNO and nNO in smoking OSA were close to the normal range.
Interestingly, we found that after sleep nNO significantly increased than before sleep nNO in OSA group and health controls in our study. The increase in OSA patients was a little more than that of control subjects, but still did not reach a significant difference. It is not clear whether NO output has circadian variation. Dias [36] observed the concentration of exhaled NO increased from early morning to midday to late afternoon, which suggest a possible circadian relationship. But O’Hacken et al. [37] measured exhaled NO once an hour and found no significant temporal pattern over a 24-h period. Chatkin et al. [38] showed nNO concentration was negatively associated with nasal air-flow, and O’Hearn found increase in exhaled NO concentration after daytime awake voluntary hypoventilation (6.8 ± 1.4 L/min) [37]. So, the confounder trans-nasal minute ventilation need to be checked before determining whether nNO can be used to reflect upper-airway inflammation in OSA patients.
Limitations
There were some limitations in this study. Firstly, the sample size was small and only include adult male; further studies are needed to confirm whether the results are applicable to female. Secondly, we did not perform nasal sinus imageological examination and bronchial provocation test; the exclusion of rhinitis, nasosinusitis, and asthma was mainly depended on history and clinical manifestation. Thirdly, nasal minute ventilation was not measured, so it was unknown whether nNO levels affected by nasal ventilation or not. Fourthly, nNO has not been widely used in clinical practice and without normal value range in China. But, the result of healthy controls in our study was very similar with that of other research (446.76 ± 133.63 ppb) [13].
Conclusion
Patients of OSA have non-symptom neutrophic rhinitis, with higher nNO concentration. Nasal NO positively correlated with OSA severity and rhinitis, suggest nNO is a promising marker of nasal inflammation in non-smoking OSA. Further studies are needed to better understand the effect of CPAP therapy on nNO and the relationship between nNO and CPAP compliance.
Abbreviations
- AHI:
-
Apnea hypopnea index
- ATS/ERS:
-
American Thoracic Society/European Respiratory Society
- BMI:
-
Body mass index
- CPAP:
-
Continuous positive airway pressure
- CV:
-
Coefficients of variation
- ESS:
-
Epworth Sleepiness Scale
- FeNO:
-
Fractional exhaled nitric oxide
- FEV1:
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- nNO:
-
Nasal nitric oxide
- OSA:
-
Obstructive sleep apnea
- PSG:
-
Polysomnography
- PUMCH:
-
Peking Union Medical College Hospital
- SpO2 :
-
Pulse arterial oxygen saturation
- TST SpO2 < 90%:
-
Total sleep time with SpO2 < 90%
- ROC:
-
Receiver operating characteristic curve
References
Bikov A, Hull JH, Kunos L (2016) Exhaled breath analysis, a simple tool to study the pathophysiology of obstructive sleep apnoea. Sleep Med Rev 27:1–8. https://doi.org/10.1016/j.smrv.2015.07.005
Munakata M (2012) Exhaled nitric oxide (FeNO) as a non-invasive marker of airway inflammation. Allergol Int 61(3):365–372. https://doi.org/10.2332/allergolint.12-RAI-0461
Zhang D, Luo J, Qiao Y, Xiao Y, Huang R, Zhong X (2017) Measurement of exhaled nitric oxide concentration in patients with obstructive sleep apnea: a meta-analysis. Medicine (Baltimore) 96(12):e6429. https://doi.org/10.1097/MD.0000000000006429
Williamson PA, Vaidyanathan S, Clearie K, Stewart M, Lipworth BJ (2010) Relationship between fractional exhaled nitric oxide and nasal nitric oxide in airways disease. Ann Allergy Asthma Immunol 105(2):162–167. https://doi.org/10.1016/j.anai.2010.05.014
Jorissen M, Lefevere L, Willems T (2001) Nasal nitric oxide. Allergy 56(11):1026–1033
Gut G, Tauman R, Greenfeld M, Armoni-Domany K, Sivan Y (2016) Nasal nitric oxide in sleep-disordered breathing in children. Sleep Breath 20(1):303–308. https://doi.org/10.1007/s11325-015-1189-8
Liu D, Huang Z, Huang Y, Yi X, Chen X (2015) Measurement of nasal and fractional exhaled nitric oxide in children with upper airway inflammatory disease: preliminary results. Int J Pediatr Otorhinolaryngol 79(12):2308–2311. https://doi.org/10.1016/j.ijporl.2015.10.033
Huang Y, Zou Y, Mai F, Zhang X, Liu Y, Lin X (2016) Sleep-disordered breathing children: measurement of nasal nitric oxide and fractional exhaled nitric oxide. HNO 64(3):169–174. https://doi.org/10.1007/s00106-016-0120-3
Olopade CO, Christon JA, Zakkar M, Hua C, Swedler WI, Scheff PA, Rubinstein I (1997) Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest 111(6):1500–1504
Petrosyan M, Perraki E, Simoes D, Koutsourelakis I, Vagiakis E, Roussos C, Gratziou C (2008) Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath 12(3):207–215. https://doi.org/10.1007/s11325-007-0160-8
Hamada S, Tatsumi S, Kobayashi Y, Yasuba H (2017) Nasal nitric oxide improved by continuous positive airway pressure therapy for upper airway inflammation in obstructive sleep apnea. Sleep Breath 21(2):405–410. https://doi.org/10.1007/s11325-016-1431-z
American Thoracic Society; European Respiratory Society (2005) ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 171(8):912–930. https://doi.org/10.1164/rccm.200406-710ST
Gelardi M, Abbattista G, Quaranta VN, Quaranta N, Seccia V, Buttafava S, Frati F, Ciprandi G (2016) Standardization procedure for the nasal nitric oxide measurement method using Niox MINO® and the tidal-breathing technique with velum-closure. J Biol Regul Homeost Agents 30(3):853–858
Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL and Vaughn BV for the American Academy of Sleep Medicine (2016) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.3. American Academy of Sleep Medicine, Darien. http://www.aasmnet.org
Devouassoux G, Lévy P, Rossini E, Pin I, Fior-Gozlan M, Henry M, Seigneurin D, Pépin JL (2007) Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J Allergy Clin Immunol 119(3):597–603. https://doi.org/10.1016/j.jaci.2006.11.638
Depalo A, Carpagnano GE, Spanevello A, Sabato R, Cagnazzo MG, Gramiccioni C, Foschino-Barbaro MP (2008) Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J Intern Med 263(1):70–78
Lundberg JO, Farkas-Szallasi T, Weitzberg E, Rinder J, Lidholm J, Anggåard A, Hökfelt T, Lundberg JM, Alving K (1995) High nitric oxide production in human paranasal sinuses. Nat Med 1(4):370–373
Lundberg JO, Weitzberg E (1999) Nasal nitric oxide in man. Thorax 54(10):947–952
Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW (2013) Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med 188(8):913–922. https://doi.org/10.1164/rccm.201301-0059CI
Struben VM, Wieringa MH, Feenstra L, de Jongste JC (2006) Nasal nitric oxide and nasal allergy. Allergy 61(6):665–670. https://doi.org/10.1111/j.1398-9995.2006.01096.x
Collins SA, Gove K, Walker W, Lucas JS (2014) Nasal nitric oxide screening for primary ciliary dyskinesia: systematic review and meta-analysis. Eur Respir J 44(6):1589–1599. https://doi.org/10.1183/09031936.00088614
Rubinstein I (1995) Nasal inflammation in patients with obstructive sleep apnea. Laryngoscope 105(2):175–177
Kao LT, Hung SH, Lin HC, Liu CK, Huang HM, Wu CS (2016) Obstructive sleep apnea and the subsequent risk of chronic rhinosinusitis: a population-based study. Sci Rep 6:20786. https://doi.org/10.1038/srep20786
Inancli HM, Enoz M (2010) Obstructive sleep apnea syndrome and upper airway inflammation. Recent Patents Inflamm Allergy Drug Discov 4(1):54–57
Xie Q, Nathan C (1994) The high-output nitric oxide pathway: role and regulation. J Leukoc Biol 56(5):576–582
Shadan FF, Jalowayski AA, Fahrenholz J, Kline LE, Dawson A (2005) Nasal cytology: a marker of clinically silent inflammation in patients with obstructive sleep apnea and a predictor of noncompliance with nasal CPAP therapy. J Clin Sleep Med 1(3):266–270
Li Y, Chongsuvivatwong V, Geater A, Liu A (2009) Exhaled breath condensate cytokine level as a diagnostic tool for obstructive sleep apnea syndrome. Sleep Med 10(1):95–103. https://doi.org/10.1016/j.sleep.2007.11.013
Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ (2002) Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest 122(4):1162–1167
Carpagnano GE, Spanevello A, Sabato R, Depalo A, Palladino GP, Bergantino L, Foschino BMP (2010) Systemic and airway inflammation in sleep apnea and obesity: the role of ICAM-1 and IL-8. Transl Res 155(1):35–43. https://doi.org/10.1016/j.trsl.2009.09.004
Oliver KM, Taylor CT, Cummins EP (2009) Hypoxia. Regulation of NFkappaB signalling during inflammation: the role of hydroxylases. Arthritis Res Ther 11(1):215. https://doi.org/10.1186/ar2575
Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I (2004) Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J 18(15):1897–1899. https://doi.org/10.1096/fj.04-1506fje
Vicente E, Marin JM, Carrizo SJ, Osuna CS, González R, Marin-Oto M, Forner M, Vicente P, Cubero P, Gil AV, Soler X (2016) Upper airway and systemic inflammation in obstructive sleep apnoea. Eur Respir J 48(4):1108–1117. https://doi.org/10.1183/13993003.00234-2016
Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ (1995) Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med 152(2):609–612. https://doi.org/10.1164/ajrccm.152.2.7543345
Hoyt JC, Robbins RA, Habib M, Springall DR, Buttery LD, Polak JM, Barnes PJ (2003) Cigarette smoke decreases inducible nitric oxide synthase in lung epithelial cells. Exp Lung Res 29(1):17–28
Assreuy J, Cunha FQ, Liew FY, Moncada S (1993) Feedback inhibition of nitric oxide synthase activity by nitric oxide. Br J Pharmacol 108(3):833–837
Dias AR, Martinho C, Teixeira J et al (2010) Circadian pattern of exhaled nitric oxide in a group of obstructive sleep apnoea patients and healthy subjects. J Sleep Res 19(1 Pt 2):164
O’Hearn DJ, Giraud GD, Sippel JM, Edwards C, Chan B, Holden WE (2007) Exhaled nasal nitric oxide output is reduced in humans at night during the sleep period. Respir Physiol Neurobiol 156(1):94–101. https://doi.org/10.1016/j.resp.2006.08.002
Chatkin JM, Qian W, McClean PA, Zamel N, Haight J, Silkoff P (1999) Nitric oxide accumulation in the nonventilated nasal cavity. Arch Otolaryngol Head Neck Surg 125(6):682–685
Acknowledgements
The authors would like to thank Hairong Zhang, Shuning Li, Juan Chen, and Yong Chen for their help in collecting data and performing nasal lavage cellular classification.
Funding
This work was supported by National Natural Science Foundation of China (81570085).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. No identifying information about participants is available in this article.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the ethics committee of Peking Union Medical College Hospital (protocol number ZS-1107).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhang, D., Xiao, Y., Luo, J. et al. Measurement of fractional exhaled nitric oxide and nasal nitric oxide in male patients with obstructive sleep apnea. Sleep Breath 23, 785–793 (2019). https://doi.org/10.1007/s11325-018-1760-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-018-1760-1