Abstract
Purpose
We aimed to determine the test–retest repeatability of quantitative metrics based on the Patlak slope (PS) versus the standardized uptake value (SUV) among lesions and normal organs on oncologic [18F]FDG-PET/CT.
Procedures
This prospective, single-center study enrolled adults undergoing standard-of-care oncologic [18F]FDG-PET/CTs. Early (35–50 min post-injection) and late (75–90 min post-injection) SUV and PS images were reconstructed from dynamic whole-body PET data. Repeat imaging occurred within 7 days. Relevant quantitative metrics were extracted from lesions and normal organs. Repeatability was assessed via mean test–retest percent changes [T-RT %Δ], within-subject coefficients of variation (wCVs), and intra-class correlation coefficients (ICCs).
Results
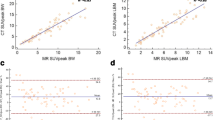
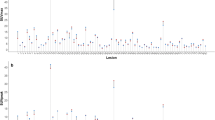
Nine subjects (mean age, 61.7 ± 6.2 years; 6 females) completed the test–retest protocol. Four subjects collectively had 17 [18F]FDG-avid lesions. Lesion wCVs were higher (i.e., worse repeatability) for PS-early-max (16.2%) and PS-early-peak (15.6%) than for SUV-early-max (8.9%) and SUV-early-peak (8.1%), with similar early metric ICCs (0.95–0.98). Lesion wCVs were similar for PS-late-max (8.5%) and PS-late-peak (6.4%) relative to SUV-late-max (9.7%) and SUV-late-peak (7.2%), with similar late metric ICCs (0.93–0.98). There was a significant bias toward higher retest SUV and PS values in the lesion analysis (T-RT %Δ [95% CI]: SUV-late-max, 10.0% [2.6%, 17.0%]; PS-late-max, 20.4% [14.3%, 26.4%]) but not in the normal organ analysis.
Conclusions
Among [18F]FDG-avid lesions, the repeatability of PS-based metrics is similar to equivalent SUV-based metrics at late post-injection time points, indicating that PS-based metrics may be suitable for tracking response to oncologic therapies. However, further validation is required in light of our study’s limitations, including small sample size and bias toward higher retest values for some metrics.






Similar content being viewed by others
Data Availability
The data supporting the findings of this study, if not already provided in the manuscript or supplemental materials, are available from the corresponding author by reasonable request.
References
Keyes JW (1995) SUV: Standard uptake or silly useless value? J Nucl Med 36:1836–1839
Beaulieu S, Kinahan P, Tseng J et al (2003) SUV varies with time after injection in 18F-FDG PET of breast cancer: Characterization and method to adjust for time differences. J Nucl Med 44:1044–1050
Lee JW, Kim SK, Lee SM, Moon SH, Kim TS (2011) Detection of hepatic metastases using dual-time-point FDG PET/CT scans in patients with colorectal cancer. Mol Imaging Biol 13:565–572
Rahmim A, Lodge MA, Karakatsanis NA et al (2019) Dynamic whole-body PET imaging: principles, potentials and applications. Eur J Nucl Med Mol Imaging 46:501–518
Zasadny KR, Wahl RL (1996) Enhanced FDG-PET tumor imaging with correlation-coefficient filtered influx-constant images. J Nucl Med 37:371–374
Verhaeghe J, Gravel P, Mio R et al (2010) Motion compensation for fully 4D PET reconstruction using PET superset data. Phys Med Biol 55:4063–4082
Kinahan PE, Perlman ES, Sunderland JJ et al (2020) The QIBA profile for FDG PET/CT as an imaging biomarker measuring response to cancer therapy. Radiology 294:647–657
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S-S150
Tao Y, Peng Z, Krishnan A, Zhou XS (2011) Robust learning-based parsing and annotation of medical radiographs. IEEE Trans Med Imaging 30:338–350
Martin Bland J, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327:307–310
Koo TK, Li MY (2016) A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 15:155–163
Chirindel A, Alluri KC, Tahari AK et al (2015) Liver standardized uptake value corrected for lean body mass at FDG PET/CT: Effect of FDG uptake time. Clin Nucl Med 40:e17–e22
Kuwabara H, Gjedde A (1991) Measurements of glucose phosphorylation with FDG and PET are not reduced by dephosphorylation of FDG-6-phosphate. J Nucl Med 32:692–698
Minn H, Zasadny KR, Quint LE, Wahl RL (1995) Lung cancer: Reproducibility of quantitative measurements for evaluating 2-[F-18]-fluoro-2-deoxy-D-glucose uptake at PET. Radiology 196:167–173
Weber WA, Ziegler SI, Thödtmann R, Hanauske AR, Schwaiger M (1999) Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med 40:1771–1777
Naganawa M, Gallezot JD, Shah V et al (2020) Assessment of population-based input functions for Patlak imaging of whole body dynamic 18F-FDG PET. EJNMMI Phys 7:67
Dias AH, Smith AM, Shah V, Pigg D, Gormsen LC, Munk OL (2022) Clinical validation of a population-based input function for 20-min dynamic whole-body 18F-FDG multiparametric PET imaging. EJNMMI Phys 9:60
Acknowledgements
There are no relevant acknowledgements.
Funding
This work was supported by a research grant from Siemens Healthineers to Washington University.
Author information
Authors and Affiliations
Contributions
S.I. – acquisition, analysis, and interpretation of data; manuscript revision
R.L. – acquisition, analysis, and interpretation of data; manuscript revision
S.A. – conceptualization; manuscript review/feedback
A.M.S. – conceptualization; manuscript review/feedback
R.L.W. – conceptualization; manuscript revision
T.J.F. – conceptualization; acquisition, analysis, and interpretation of data; manuscript drafting
Corresponding author
Ethics declarations
Conflict of Interest
This work was supported by a research grant from Siemens Healthineers to Washington University. This grant included salary support for Dr. Fraum. Note that all subjects were imaged on a Siemens Healthineers PET/CT scanner and that two authors (Dr. Ashrafinia, Dr. Smith) are full-time employees of Siemens Medical Solutions USA, Inc. Dr. Ashrafinia and Dr. Smith participated in the initial study design, provided occasional technical support, and critically reviewed the manuscript. However, all data collection/analysis and manuscript drafting was performed exclusively by the authors from Washington University, independent of any individuals affiliated with Siemens.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ince, S., Laforest, R., Ashrafinia, S. et al. Test–Retest Repeatability of Patlak Slopes versus Standardized Uptake Values for Hypermetabolic Lesions and Normal Organs in an Oncologic PET/CT Population. Mol Imaging Biol 26, 284–293 (2024). https://doi.org/10.1007/s11307-024-01909-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-024-01909-x