Abstract
Purpose
Idiopathic pulmonary fibrosis (IPF) is a destructive lung disease with a poor prognosis, an unpredictable clinical course, and inadequate therapies. There are currently no measures of disease activity to guide clinicians making treatment decisions. The aim of this study was to develop a PET probe to identify lung fibrogenesis using a pre-clinical model of pulmonary fibrosis, with potential for translation into clinical use to predict disease progression and inform treatment decisions.
Methods
Eight novel allysine-targeting chelators, PIF-1, PIF-2, …, PIF-8, with different aldehyde-reactive moieties were designed, synthesized, and radiolabeled with gallium-68 or copper-64. PET probe performance was assessed in C57BL/6J male mice 2 weeks after intratracheal bleomycin challenge and in naïve mice by dynamic PET/MR imaging and with biodistribution at 90 min post injection. Lung hydroxyproline and allysine were quantified ex vivo and histological staining for fibrosis and aldehyde was performed.
Results
In vivo screening of probes identified 68GaPIF-3 and 68GaPIF-7 as probes with high uptake in injured lung, high uptake in injured lung versus normal lung, and high uptake in injured lung versus adjacent liver and heart tissue. A crossover, intra-animal PET/MR imaging study of 68GaPIF-3 and 68GaPIF-7 confirmed 68GaPIF-7 as the superior probe. Specificity for fibrogenesis was confirmed in a crossover, intra-animal PET/MR imaging study with 68GaPIF-7 and a non-binding control compound, 68GaPIF-Ctrl. Substituting copper-64 for gallium-68 did not affect lung uptake or specificity indicating that either isotope could be used.
Conclusion
A series of allysine-reactive PET probes with variations in the aldehyde-reactive moiety were evaluated in a pre-clinical model of lung fibrosis. The hydrazine-bearing probe, 68GaPIF-7, exhibited the highest uptake in fibrogenic lung, low uptake in surrounding liver or heart tissue, and low lung uptake in healthy mice and should be considered for further clinical translation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a persistent, progressive scarring lung disease which carries a poor prognosis and has limited therapeutic options [1]. Current therapies only slow the rate of lung function decline and have significant associated side effects [2, 3]. One of the most challenging aspects of diagnosing IPF is that there are currently no disease activity measures, which could predict the likelihood of a particular disease trajectory and could inform practitioners about when to initiate antifibrotic therapies [4]. There is also a critical need for disease activity markers to facilitate decision-making about treatment effectiveness and to inform future clinical trial design and decisions about efficacy of new drug therapies [5]. Currently, there are 2 FDA-approved therapies for IPF, but neither halt progression of disease or reverse established fibrosis in the lungs [2, 3]. A disease activity marker would be incredibly useful, and molecular probes used through non-invasive PET imaging techniques have great potential to provide these critical tools [6].
Pulmonary fibrosis is characterized by excessive activation of collagen-producing cells, myofibroblasts, in the lungs of affected patients. These myofibroblasts deposit large quantities of extracellular matrix (ECM), predominantly collagens, in the lungs. As a result, the lungs become stiff and scarred and unable to perform their normal functions. Collagen maturation is initiated by the lysyl oxidase (LOX) enzymatic oxidation of lysine residues in the collagen helices to corresponding allysine residues which can then undergo condensation reactions leading to a cross-linked fibrillar matrix [7]. Increased LOX activity is a hallmark of disease activity in IPF [8].
Previously, we reported MRI and PET probes that bind to allysine moieties in fibrotic lung tissue [9, 10]. However, MRI is limited in the lung and the PET probe 68Ga-NODAGA-indole displayed substantial hepatobiliary elimination. Because IPF is a lower lobe predominant disease, high liver uptake would limit the ability to delineate fibrogenesis at the base of the lungs. The goal of this study was to identify a new allysine-specific probe with high uptake in injured, fibrotic lung but low uptake in healthy lung and low uptake in surrounding background tissues. Here, we describe the synthesis of a library of highly hydrophilic compounds bearing various aldehyde-reacting moieties and their evaluation in the bleomycin model of pulmonary fibrosis to identify an optimized pulmonary fibrogenesis probe.
Methods
Probe Synthesis
Radiolabeling was performed by adding 68GaCl3 or 64CuCl2 to precursor in sodium acetate buffer, followed by heating; no further purification was required. Detailed synthetic procedures for the precursors and labeling conditions can be found in ESI.
Animal Studies
Animal studies followed ARRIVE guidelines [11]. A total of 145 of mice were used for these experiments. Pulmonary fibrosis was induced in 10–12-week-old male C57/BL6 mice (Charles River Laboratories, Wilmington MA) by administering a single intratracheal dose of bleomycin (Fresenius Kabi, Lake Zurich, IL, 1.2 unit/kg in 50 μL sterile phosphate-buffered saline). Naïve mice were used as healthy controls. Animals who lost less than 5% of their body weight by day 13 were excluded from the experiment. Imaging and biodistribution studies were performed 13 to 15 days after bleomycin instillation.
Immunohistochemical Staining
Lung tissue was obtained from patients at Massachusetts General Hospital (MGH) diagnosed with IPF at time of explant and from non-fibrotic healthy control patients undergoing video-assisted thoracoscopic surgery for non-fibrotic lesion sampling. Lung tissues from mice were collected after imaging and inflation with methacarn. Human and mouse lung tissues were fixed in methacarn for 10 min, transferred into 60% ethanol for storage, imbedded in paraffin, and sectioned for staining. Trichrome and H&E staining was performed through the MGH Histopathology Core. Aldehyde staining was performed on 7-μm-thick sections according to a dinitrophenylhydrazine reactivity assay as previously described [12].
Ex Vivo Analyses
Lung hydroxyproline concentration was measured using a colorimetric assay as described previously [13]. Lung allysine was measured as reported previously [14], with some modifications as described in the ESI.
Biodistribution
Ninety minutes after intravenous administration of probe, animals were euthanized and the kidneys, liver, heart, spleen, pancreas, urine, blood, lungs, stomach, intestines, and tail were harvested and weighed, and radioactivity in each tissue was measured on a gamma counter (Wizard2Auto Gamma, Perkin Elmer) and decay corrected. Probe distribution is presented as percent ID/gram for all organs and as percent ID/lung (left lung).
PET-MR Imaging
Mice were simultaneously imaged with PET and MRI in a 4.7 T MRI scanner equipped with a PET insert (Bruker, Billerica, MA) following tail vein administration of a probe. Image acquisition and reconstruction details are found in the ESI.
PET-MR Image Analysis
PET image analysis was performed using the AMIDE software package by a reader who had 8 years of experience and who was blinded to the animal groups or the injected probe. Volumes of interest (VOIs) over main organs including the lung, liver, heart, and kidney were defined from the anatomic MR images and copied to the fused PET images. Lung VOIs were manually drawn over the right lung excluding airways and large vessels. Time-activity curves were calculated from these VOIs. Results were expressed as a percentage of injected dose per cubic centimeter of tissue (%ID/cc).
Statistics
Data were expressed as mean ± SEM. Differences between two groups were compared using two-sided unpaired t-test; differences between more than two groups were compared by ANOVA, followed by Tukey’s post hoc test; intra-animal comparisons were performed using a paired t-test. p ≤ 0.05 was considered significant.
Results
Allysine Is a Marker of Active Fibrogenesis and an Abundant Target in Both Human Disease and Mouse Models
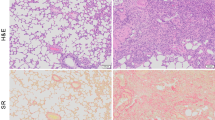
The fibrotic cascade involves upregulation of LOX, oxidation of collagen lysine side chains, and ultimate formation of branched cross-linked aggregates (Fig. 1A). Dinitrophenylhydrazine (DNPH) staining shows an abundance of tissue aldehyde in human IPF lung (Fig. 1B) and in bleomycin-injured mouse lung (Fig. 1C), but not in normal lung tissue. Hematoxylin-eosin staining shows architectural distortion in both human IPF and the bleomycin-injured lung, while Masson-Trichrome staining indicates the presence of fibrosis in the diseased lungs.
Allysine is a marker of active fibrogenesis and an abundant target in both human disease and mouse models. A Oxidation of collagen by lysyl oxidases (LOX) to give aldehyde-containing allysine residues that slowly form crosslinks with other collagens. B Hematoxylin and eosin (HE) staining and Masson’s trichrome staining of normal and IPF lungs demonstrate extensive collagen deposition in the fibrotic lung. Aldehyde staining (Allysine) with DNPH demonstrates abundant accumulation in the fibrotic lung. C Hematoxylin and eosin and Masson’s trichrome staining of naïve and bleomycin mouse lungs also demonstrate extensive collagen deposition in the fibrotic lung. Aldehyde staining (pink/brown) with DNPH demonstrates abundant accumulation in the fibrotic lung
Design and Synthesis of Allysine-Targeted PET Probes
Eight precursor compounds were designed and share common structural motifs (Fig. 2A): a NOTA-derived chelator for incorporation of a radiometal, a short PEGn linker (n = 3 for 68GaPIF-2–8, n = 4 for 68GaPIF-1) to increase hydrophilicity in order to minimize hepatobiliary clearance, and an aldehyde-reactive moiety for targeting allysine. Eight different targeting moieties were included which can react with aldehydes via hydrazone formation (PIF-1, PIF-5, PIF-7), Pictet-Spengler ligation (PIF-2), Knoevenagel condensation (PIF-3, PIF-4, PIF-6), or oxime formation (PIF-8). The reactivity of the radiolabeled probes was verified by adding a model aldehyde, pyridine-2-carbaldehyde, giving rise to a new peak of the conjugated product in the radio-HPLC trace (Fig. 2B).
Screening of PET probes demonstrated 68Ga-PIF7 and 68Ga-PIF3 as potential development candidates. A Chemical structures of the 8 PET probes synthesized for testing in the bleomycin model of pulmonary fibrosis. B HPLC traces demonstrating radiochemical purity of 68Ga-PIF7 and its reactivity with a model aldehyde, pyridine-2-carbaldehyde. C Schematic of experimental design. D Comparison of probe lung uptake (%ID/g) in bleomycin-injured animals versus bleomycin injured to naïve lung uptake ratio. E Comparison of probe lung uptake (%ID/g) in bleomycin-injured animals versus lung to liver ratio of probe uptake in bleomycin-injured animals. F Comparison of probe lung uptake (%ID/g) in bleomycin-injured animals versus lung to heart ratio of probe uptake in bleomycin-injured animals
Comparison of Probe Biodistribution in Bleomycin-Injured and Naïve Mice Identifies 68GaPIF-3 and 68GaPIF-7 as Lead Candidates
We compared the 90-min biodistribution of 8 different 68Ga-labeled probes in bleomycin-injured mice at day 14 following bleomycin instillation and in naïve mice (Fig. 2C). Figure 2D compares the probes according to their lung uptake (%ID/g) in bleomycin-injured animals plotted versus the ratio of lung uptake between bleomycin-injured and naïve healthy controls. Comparisons were also drawn between the probes based on their liver (Fig. 2E) and heart (Fig. 2F) uptake compared to their uptake in fibrotic lung. For both comparisons, 68GaPIF-3 and 68GaPIF-7 demonstrated a combination of high uptake in fibrotic lung compared to uninjured lung and low background in the liver and heart.
Pairwise Comparison Between 68GaPIF-7 and 68GaPIF-3 in PET/MR Imaging Study
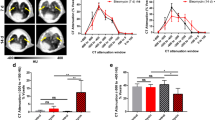
Because of the heterogeneity in the bleomycin model, we performed an intra-animal comparison of lung uptake and lung-to-background ratios for 68GaPIF-3 and 68GaPIF-7 using dynamic PET/MRI. On day 14 after bleomycin instillation, mice were injected with 68GaPIF-3 and the next day were imaged using 68GaPIF-7 (Fig. 3A). 68GaPIF-7 PET/MR revealed predominantly lung-specific uptake, with significantly lower signal from adjacent tissues, most notably the liver and heart (Fig. 3B). Visibly poorer delineation between the lung and liver was observed in the animal injected with 68GaPIF-3 (Fig. 3C). Blinded analysis of the PET data at 50–60 min post injection revealed significantly (p = 0.0022) higher lung uptake with 68GaPIF-7 (%ID/cc = 0.87 ± 0.34) than with 68GaPIF-3 (%ID/cc = 0.67 ± 0.28) consistent with the screening biodistribution study (Fig. 3D). There was no significant difference in lung-to-liver or lung-to-heart ratios between the probes (Figure S1). Because of the higher fibrotic lung uptake with 68GaPIF-7 and because of its simpler radiochemical synthesis, we selected 68GaPIF-7 for further study.
Crossover comparison PET/MR imaging study of 68Ga-PIF7 versus 68Ga-PIF3. A Schematic of experimental design with 68Ga-PIF3 PET on day 14 and 68Ga-PIF7 PET on day 15 after IT bleomycin. B From left to right: coronal MR, PET, and fused PET/MR images (55 min p.i.) of a representative bleomycin-treated animal intravenously injected with 68Ga-PIF3 on day 14. C From left to right: coronal MR, PET, and fused PET/MR images (55 min p.i.) of the same bleomycin-treated animal from B intravenously injected with 68Ga-PIF7 on day 15. D Pairwise comparison of 68Ga-PIF3 and 68Ga-PIF7 uptake (%ID/cc) in the lung. **p < 0.01, paired t-test
Dynamic PET/MR Imaging with 68GaPIF-7 in Bleomycin-Injured and in Naïve Mice
Dynamic 68GaPIF-7 PET in bleomycin-injured and naïve mice was acquired for 60 min (Fig. 4A). Blood pool (left ventricle) time-activity curves from 0 to 60 min post injection of 68GaPIF-7 are shown in Fig. 4B (naïve mice) and C (bleomycin-injured mice). 68GaPIF-7 was predominantly eliminated via the kidneys. No evidence of hepatobiliary elimination was observed, e.g., no gall bladder uptake and rapid decrease in liver signal consistent with only blood pool enhancement. Overall, > 95% of all injected activity is confined to the kidneys and urinary bladder by 60 min p.i. 68GaPIF-7 displayed a biexponential blood clearance reflective of a fast distribution phase and a slower elimination phase (Fig. 4B, C). There was no difference in distribution or elimination half-lives between naïve and bleomycin-injured mice (t1/2 distribution = 1.1 ± 0.2 min versus 0.8 ± 0.1 min, respectively, p > 0.05; t1/2 elimination = 11.8 ± 0.7 min versus 13.9 ± 0.6 min, respectively, p > 0.05). Figure 4D shows that 68GaPIF-7 (%ID/cc) lung uptake at 55 min p.i. was significantly elevated in bleomycin-injured animals compared to controls (%ID/cc = 0.52 ± 0.23 versus 0.18 ± 0.07, p = 0.0003). Figure 4E shows significantly higher lung-to-liver ratios in bleomycin-injured animals (1.82 ± 0.37) than in naïve controls (0.98 ± 0.19, p < 0.0001). Biodistribution data at 90 min p.i. showed significantly higher lung uptake in bleomycin-injured mice compared to controls when expressed either as %ID/g (0.96 ± 0.40 vs 0.27 ± 0.05, p = 0.0042) or %ID/lung (0.41 ± 0.24 vs 0.08 ± 0.01, p = 0.0227). On the other hand, there was no significant difference in probe uptake in lung-adjacent organs between bleomycin-injured mice and healthy controls (Fig. 4F). The bleomycin-injured lungs had significantly higher collagen than normal lung as assessed by hydroxyproline content (Fig. 4G: bleomycin 269 ± 136 μg/lung vs naïve 129 ± 44 μg/lung, p = 0.0137) as well as significantly higher allysine content (Fig. 4H: bleomycin 4.2 ± 1.6 nmol/lung vs naïve 2.4 ± 0.2 nmol/lung, p = 0.0453). 68GaPIF-7 probe uptake (% ID/cc) correlates with both hydroxyproline content and allysine concentration (Figure S2).
PET/MR imaging with 68Ga-PIF7. A Coronal MR, 68Ga-PIF7 PET, and fused PET/MR images of a naïve mouse (top) and of a bleomycin-injured mouse (14 days post bleomycin, bottom) acquired 55 min p.i. B Time-activity curves (%ID/cc) in the lung, heart, and liver of naïve mice. C Time-activity curves (%ID/cc) in the lung, heart, and liver of bleomycin-injured mice. D 68Ga-PIF7 lung uptake (%ID/cc) measured by PET was significantly higher in bleomycin-injured animals than in naïve animals. E PET lung-to-liver ratio was significantly higher in bleomycin-injured animals than in naïve animals. F Biodistribution of 68Ga-PIF7 in bleomycin-injured and naïve mice showing significantly higher lung uptake in bleomycin-injured mice. G Bleomycin-injured mice had significantly higher amounts of collagen in their lungs compared to naïve mice as quantified by hydroxyproline assay. H Bleomycin-injured mice had significantly higher amounts of allysine in their lungs relative to naïve mice. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, unpaired t-test
Specificity of 68GaPIF-7 for Pulmonary Fibrosis
To confirm the specificity of 68GaPIF-7 probe for fibrotic lung tissue, we synthesized a structurally similar control probe, 68GaPIF-Ctrl, that does not bind to allysine (Fig. 5A). Figure 5B shows 90-min biodistribution data of 68GaPIF-Ctrl in bleomycin-injured and naïve mice; there was no significant difference in lung uptake between these groups (%ID/g = 0.07 ± 0.04 vs 0.06 ± 0.08, respectively, p > 0.05). We performed a pairwise imaging comparison in bleomycin-injured mice with 68GaPIF-7 administered on day 14 after bleomycin instillation and 68GaPIF-Ctrl administered the following day. Figure 5C top panel shows representative axial PET, MR, and fused PET/MR images 55 min p.i. of 68GaPIF-7 with high lung uptake as expected. Figure 5C bottom shows the 68GaPIF-Ctrl PET image in the same mouse obtained at the same time point post injection and demonstrates no appreciable lung uptake of the control probe.
Biodistribution of 68Ga-PIFCtrl control probe in bleomycin-injured and naïve mice and comparison to 68Ga-PIF7 in a PET/MR imaging study. A Chemical structure of 68Ga-PIFCtrl control probe. B Biodistribution of 68Ga-PIFCtrl in bleomycin-injured and naïve mice showing no significant differences in organ uptake in bleomycin-injured mice compared to naïve mice. C Axial MR, PET, and fused PET/MRI images of a bleomycin-injured mouse (14 days post injury) acquired 55 min p.i. of 68Ga-PIF7 (top) and the next day acquired 55 min p.i. of 68Ga-PIFCtrl (bottom) showing high selective lung uptake with 68Ga-PIF7 but not 68Ga-PIFCtrl
Comparison with 64CuPIF-7 in PET/MR Imaging Study
We next tested whether substituting 64Cu for 68Ga would alter the distribution and lung uptake properties, since divalent Cu2+ confers a different overall charge on the molecule. 64CuPIF-7 (Fig. 6A) exhibited rapid biexponential blood elimination in naïve mice, as well as rapid clearance from liver and lung (Fig. 6B). Blood clearance was somewhat faster for both the distribution (0.92 ± 0.28 min for 64CuPIF-7 vs 1.1 ± 0.2 min for 68GaPIF-7) and the elimination (7.1 ± 1.4 min for 64CuPIF-7 vs 11.8 ± 0.7 min for 68GaPIF-7) phases in naïve animals injected with 64CuPIF-7 than 68GaPIF-7. The 90-min biodistribution (Fig. 6C) of 64CuPIF-7 was similar between naïve and bleomycin-injured mice except in the lung where the lung uptake was higher in bleomycin-injured mice (0.94 ± 0.39 vs 0.47 ± 0.19, p = 0.0306). There was no evidence of off-target accumulation. Figure 6D shows MR, PET, and fused PET/MR images of a bleomycin-injured mouse (top) and a naïve mouse (bottom) at 55 min post 64CuPIF-7. Figure 6E shows significantly higher lung uptake in bleomycin-injured mice compared to naïve mice (%ID/cc = 0.71 ± 0.30 vs %ID/cc = 0.22 ± 0.07, p = 0.0097), as well as a significantly higher signal in the heart (blood pool) in injured mice (%ID/cc = 0.32 ± 0.13 vs %ID/cc = 0.14 ± 0.04, p = 0.0194), with the latter being likely reflective of either a slower clearance of the probe in the sick animals, or a partial volume effect from adjacent lung tissue.
64Cu-PIF7 PET/MR imaging and biodistribution in bleomycin-injured and naïve mice. A Chemical structure of 64Cu-PIF7 probe. B Time-activity curves (%ID/cc) in the lung (circles, solid line), heart/blood pool (square, dashed line), and liver (triangles, dotted line) of naive mice and bleomycin-injured mice. C Biodistribution of 64Cu-PIF7 in naïve and bleomycin-injured mice at 90 min p.i. showing significantly higher lung uptake in bleomycin-injured lungs compared to naïve lungs. D Coronal MR, 64Cu-PIF7 PET, and fused PET/MR images of a naïve mouse (top) and of a bleomycin-injured mouse (14 days post bleomycin, bottom) acquired 55 min p.i. E Comparison of the lung, blood pool, and liver uptake (%ID/cc) measured by PET of 64Cu-PIF7 in bleomycin-injured and naïve mice. *p < 0.05, **p < 0.01, unpaired t-test
64CuPIF-7 was compared to 68GaPIF-7 in a pairwise comparison study in bleomycin-injured mice (see Figure S3 in ESI). On day 14 after bleomycin injury, mice were imaged with 68GaPIF-7, and then 2 days later, the mice were imaged again with 64CuPIF-7. A similar distribution pattern was observed with both probes, but 64CuPIF-7 exhibited a small, but significant increase in lung uptake compared to 68GaPIF-7 (0.71 ± 0.30 %ID/cc vs 0.47 ± 0.24, p = 0.034). However, 64CuPIF-7 also showed significantly higher liver uptake compared to 68GaPIF-7 (%ID/cc = 0.48 ± 0.24 vs %ID/cc = 0.25 ± 0.11, p = 0.0109). Normalizing lung uptake to either the liver or heart showed no significant difference between the two probes.
Discussion
Current therapeutic options for IPF are inadequate due to limited efficacy and significant side effects. Identifying patients with active disease would allow clinicians to therapeutically target those who would benefit most from treatment and would improve care by recognizing which patients are responding to current and future therapies. In clinical practice, decline in pulmonary function over time has been shown to best predict mortality in IPF [15]. However, once lung function has been lost, there are no therapies available to reverse fibrosis or improve lung function. Therefore, it is critical to know when to initiate therapy to prevent loss of lung function in a proactive manner. Early diagnosis of fibrogenic activity would allow current disease-slowing treatments to begin sooner which may significantly prolong life expectancy of affected patients. A disease activity imaging biomarker would also be helpful to enrich clinical trials with patients likely to meet pre-specified endpoints of disease progression and assess for treatment response. Finally, identifying patients with active disease would enable improved IPF prognostication and allow clinicians to individualize care plans accordingly, including referral of those identified as being at high risk for disease progression for lung transplantation evaluation.
We recently identified allysine as a target for imaging fibrogenesis [16]. Extracellular allysine is a much more dynamic target than total collagen to reflect disease activity. In the absence of disease, allysine is present at low levels, but during fibrogenesis, the action of lysyl oxidase on collagen converts lysine to allysine. However, if the fibrosing insult is halted, all the allysine residues eventually become consumed as chemical crosslinks. A prior study demonstrated the potential for allysine imaging to recognize active pulmonary disease, but the utility of that earlier probe was limited by high hepatobiliary uptake, important because of the preference for IPF to localize in the lower lobes of the lungs [16].
Here, we synthesized and screened a library of 8 different allysine-binding 68Ga-based PET probes to select for high lung uptake in fibrotic lung, specific uptake in fibrotic lung versus naïve lung, and high lung-to-liver ratio. Pairwise crossover imaging studies confirmed that 68GaPIF-7 performed best. Because allysine is present at high micromolar concentrations during fibrosis [9, 14], a blocking study was impractical; instead, we compared lung uptake to a structurally similar control probe, 68GaPIF-Ctrl, to demonstrate specific binding.
We also prepared 64CuPIF-7 and compared its performance to 68GaPIF-7. The commercial availability of 68Ge/68Ga generators has resulted in an increase in 68Ga tracers used clinically. 64Cu is an alternative to 68Ga and has lower positron energy than 68Ga (Emax = 0.58 keV vs Emax = 1.90 MeV for 68Ga) which results in shorter positron range (Rmax < 3 mm for 64Cu and Rmax ~ 9 mm for 68Ga) and higher spatial resolution PET images. The much longer half-life of 64Cu (12.7 h) enables shipping over a much larger distance. We found that both 64CuPIF-7 and 68GaPIF-7 performed equally well in terms of lung uptake and lung-to-background ratios.
There is growing interest in developing tools to image fibrosis. Molecular imaging of fibroblast activating protein has been studied in multiple interstitial lung diseases with somewhat conflicting results [17, 18]. 68Ga-CBP8 and 64Cu-CBP7 target type 1 collagen and have been deployed in animal models, in explanted human lung tissue and in patients with IPF [19,20,21]. Chemokines CXCR4 and CXCL2 have also been targeted as markers of fibrosis or inflammation in IPF [22, 23]. Finally, integrin αvβ6, which is upregulated in the epithelium in pulmonary fibrosis, is another target for molecular imaging and as a target for novel antifibrotics in clinical development [24]. The features which distinguish our 68Ga-PIF7 molecular probe from those probes are that it targets one of the last intermediates in the fibrotic cascade, short-lived oxidized collagen species, thus potentially offering real-time monitoring of fibrogenesis. This ability would allow clinicians to individualize care plans accordingly, including referral of those identified as being at high risk for disease progression for lung transplantation evaluation, while minimizing dosing in patients with slower progression or discontinuation of futile therapy altogether in non-responding individuals.
There are some limitations to our study. The purpose of this study was to screen different probes to select an optimized candidate. Further validations are required to show that 68Ga-PIF7 can report on the natural history of bleomycin-induced pulmonary fibrosis and the effect of therapeutic interventions. In the crossover studies, mice were not randomly assigned the order in which they received a probe. Because we had already collected ex vivo data with 68Ga-PIF7, we imaged with this probe on the first day and then used the comparator probe the next day so that we could collect ex vivo data with the comparator probe. We did this in order to minimize the number of animals used for the studies, but this may have created a bias where, in this fast-progressing model, the mice may have been sicker on day 2. However, this potential bias would be in favor of the comparator probe.
In conclusion, we optimized and validated a PET molecular probe which targets allysine in the fibrogenic lung. This probe has the potential to provide invaluable information in the diagnosis and treatment of patients suffering from pulmonary fibrosis.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lederer DJ, Martinez FJ (2018) Idiopathic pulmonary fibrosis. N Engl J Med 379:797–798
Richeldi L, du Bois RM, Raghu G et al (2014) Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370:2071–2082
King TE Jr, Bradford WZ, Castro-Bernardini S et al (2014) A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370:2083–2092
Ley B, Collard HR, King TE Jr (2011) Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183:431–440
Bowman WS, Echt GA, Oldham JM (2021) Biomarkers in progressive fibrosing interstitial lung disease: optimizing diagnosis, prognosis, and treatment response. Front Med (Lausanne) 8:680997
Montesi SB, Desogere P, Fuchs BC, Caravan P (2019) Molecular imaging of fibrosis: recent advances and future directions. J Clin Invest 129:24–33
Rodriguez-Pascual F, Rosell-Garcia T (2018) Lysyl oxidases: functions and disorders. J Glaucoma 27(Suppl 1):S15–S19
Chen L, Li S, Li W (2019) LOX/LOXL in pulmonary fibrosis: potential therapeutic targets. J Drug Target 27:790–796
Chen HH, Waghorn PA, Wei L et al (2017) Molecular imaging of oxidized collagen quantifies pulmonary and hepatic fibrogenesis. JCI Insight 2(11):e91506
Ning Y, Zhou IY, Roberts JD Jr et al (2022) Molecular MRI quantification of extracellular aldehyde pairs for early detection of liver fibrogenesis and response to treatment. Sci Transl Med 14:eabq6297
Percie du Sert N, Hurst V, Ahluwalia A et al (2020) The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Physiol 598:3793–3801
Zhong Y, Mahoney RC, Khatun Z et al (2022) Lysyl oxidase regulation and protein aldehydes in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol 322:L204–L223
Tager AM, LaCamera P, Shea BS et al (2008) The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 14:45–54
Waghorn PA, Oliveira BL, Jones CM, Tager AM, Caravan P (2017) High sensitivity HPLC method for determination of the allysine concentration in tissue by use of a naphthol derivative. J Chromatogr B Analyt Technol Biomed Life Sci 1064:7–13
Brown KK, Inoue Y, Flaherty KR et al (2022) Predictors of mortality in subjects with progressive fibrosing interstitial lung diseases. Respirology 27:294–300
Wahsner J, Desogere P, Abston E et al (2019) (68)Ga-NODAGA-Indole: an allysine-reactive positron emission tomography probe for molecular imaging of pulmonary fibrogenesis. J Am Chem Soc 141:5593–5596
Yang P, Fang Q, Fu Z et al (2022) Comprehensive analysis of fibroblast activation protein (FAP) expression in interstitial lung diseases (ILDs). Am J Respir Crit Care Med 207(2):160–172
Montesi SB, Horowitz JC (2022) Fibroblast activating protein: skimming the surface of molecular imaging to assess fibrotic disease activity. Am J Respir Crit Care Med 207(2):122–124
Montesi SB, Izquierdo-Garcia D, Desogere P et al (2019) Type I collagen-targeted positron emission tomography imaging in idiopathic pulmonary fibrosis: first-in-human studies. Am J Respir Crit Care Med 200:258–261
Désogère P, Tapias LF, Hariri LP et al (2017) Type I collagen-targeted PET probe for pulmonary fibrosis detection and staging in preclinical models. Sci Transl Med 9(384):eaaf4696
Désogère P, Tapias LF, Rietz TA et al (2017) Optimization of a collagen-targeted PET probe for molecular imaging of pulmonary fibrosis. J Nucl Med 58:1991–1996
Brody SL, Gunsten SP, Luehmann HP et al (2021) Chemokine receptor 2-targeted molecular imaging in pulmonary fibrosis. A clinical trial. Am J Respir Crit Care Med 203:78–89
Derlin T, Jaeger B, Jonigk D et al (2021) Clinical molecular imaging of pulmonary CXCR4 expression to predict outcome of pirfenidone treatment in idiopathic pulmonary fibrosis. Chest 159:1094–1106
Kimura RH, Wang L, Shen B et al (2019) Evaluation of integrin alphavbeta6 cystine knot PET tracers to detect cancer and idiopathic pulmonary fibrosis. Nat Commun 10:4673
Funding
This work was supported by the National Heart Lung and Blood Institute (R33HL154125 to PC, K08HL140175 to RSK), the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK121789 to PC), and the National Institutes of Health Office of the Director (OD025234, OD010650, OD023503, OD028499, and OD032138 to PC)
Author information
Authors and Affiliations
Contributions
Conception and design: S. S, R.S.K., and P.C.; data acquisition: S.S., M.D., R.S.K., J.W.-W., H.M., I.Y.Z., J.D.R., N.J.R., and Y.N.; analysis and interpretation: S.S., M.D., I.Y.Z., J.D.R., Y. N., N.J.R., R.S.K, and P.C.; drafting the manuscript: S.S., R.S.K., and M.D.; revising the manuscript for important intellectual content: S.S., R.S.K., and P.C.
Corresponding author
Ethics declarations
Ethics Approval
All experiments and procedures were performed in accordance with the National Institutes of Health’s “Guide for the Care and Use of Laboratory Animals” and were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Competing Interests
Financial interests: S.S., Y.N., and P.C. are inventors on a patent application that covers the compounds in this report. P.C. has equity in Reveal Pharmaceuticals and Collagen Medical and received consulting income from Collagen Medical. P.C. has received research funding from Janssen, Takeda, Pliant Therapeutics, and Transcode Therapeutics. All others authors declare no competing interests.
Additional information
Key Points
Question: The goal of this work was to identify an optimized PET probe to measure disease activity in idiopathic pulmonary fibrosis.
Pertinent Findings: We synthesized 8 novel allysine targeting precursors and evaluated their Ga-68 and Cu-64 complexes in a mouse model of pulmonary fibrosis. 68Ga-PIF7 and 64Cu-PIF7 showed high specificity for pulmonary fibrogenesis and high target-to-background ratios.
Implications for Patient Care: 68Ga-PIF7 and 64Cu-PIF7 are strong candidates for clinical translation.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sergey Shuvaev and Rachel S. Knipe are co-first authors.
Supplementary Information
ESM 1
(PDF 1861 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shuvaev, S., Knipe, R.S., Drummond, M. et al. Optimization of an Allysine-Targeted PET Probe for Quantifying Fibrogenesis in a Mouse Model of Pulmonary Fibrosis. Mol Imaging Biol 25, 944–953 (2023). https://doi.org/10.1007/s11307-023-01845-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-023-01845-2