Abstract
Purpose
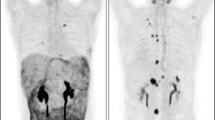
In this study, we aimed to investigate the utilization of 68Ga-FAPI PET/CT in comparison to 18FDG PET/CT to evaluate the peritoneal involvement of the gastrointestinal malignancies alongside primary lesions and other metastatic foci.
Procedures
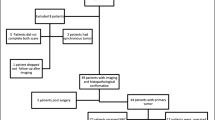
A total of 37 patients with various gastrointestinal malignancies with accompanying peritoneal involvement who underwent 68Ga-FAPI PET/CT and 18FDG PET/CT imaging between September 2020 and June 2021 were included in this retrospective study. SUVmax values of 68Ga-FAPI and 18FDG were compared according to lesion locations. Also, the lesion localization ability of both imaging was compared in patient basis.
Results
Of the 37 patients with peritoneal involvement (23 males and 14 females; median age, 62.8 ± 12.7 years), 35.1% (n = 13) had colorectal cancer, 37.8% (n = 14) gastric cancer, and 27.0% (n = 10) pancreaticobiliary cancer. While 45.9% of them were operated, the remaining did not have surgery. The mean time interval between two studies was 3.2 days (range: 2–6 days). The mean SUVmax value of peritoneal metastases (p < 0.001) was significantly higher with 68Ga-FAPI PET/CT compared to that with 18FDG PET/CT, as in primary lesions (p < 0.001), lymph node metastases (p = 0.006), liver metastases (p = 0.002), and bone metastases (p = 0.018). A total of 185 lesions was detected in the initial assessment with 18FDG PET/CT. Of the total lesions detected with 18FDG PET/CT, 5 of them were evaluated as benign lesions with 68Ga-FAPI PET/CT also in accordance with the reference standard. In addition to 180 lesions detected with 18FDG PET/CT, a total of 37 additional malignant lesions, 12 of which were peritoneal metastases, were detected with 68Ga-FAPI PET/CT.
Conclusion
68Ga-FAPI PET/CT was determined to be superior to 18FDG PET/CT in terms of detection of peritoneal involvement with high image quality as well as primary tumor and other metastatic foci. Consequently, 68Ga-FAPI PET/CT can be used as a complementary imaging modality especially for inconclusive 18FDG findings due to the lack of accuracy of 18FDG PET/CT in some of the metastatic regions, especially in the liver.



Similar content being viewed by others
References
Brizi MG, Natale L, Manfredi R, Barbaro B, Vecchioli A, Marano P (2001) Staging of pancreatic ductal adenocarcinoma with spiral CT and MRI. Rays 26(2):151–159
Low RN (2007) MR imaging of the peritoneal spread of malignancy. Abdom Imaging. https://doi.org/10.1007/s00261-007-9210-8
Kostakoglu L, Agress H Jr, Goldsmith SJ (2003) Clinical role of FDG PET in evaluation of cancer patients. Radiographics. https://doi.org/10.1148/rg.232025705
Kim SJ, Lee SW (2018) Diagnostic accuracy of 18F-FDG PET/CT for detection of peritoneal carcinomatosis: a systematic review and meta-analysis. Br J Radiol. https://doi.org/10.1259/bjr.20170519
Suzuki A, Kawano T, Takahashi N, Lee J, Nakagami Y, Miyagi E et al (2004) Value of 18F-FDG PET in the detection of peritoneal carcinomatosis. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-004-1577-y
Lopez-Lopez V, Cascales-Campos PA, Gil J, Frutos L, Andrade RJ, Fuster-Quinonero M et al (2016) Use of (18)F-FDG PET/CT in the preoperative evaluation of patients diagnosed with peritoneal carcinomatosis of ovarian origin, candidates to cytoreduction and hipec. A pending issue Eur J Radiol. https://doi.org/10.1016/j.ejrad.2016.08.006
Soyka JD, Strobel K, Veit-Haibach P, Schaefer NG, Schmid DT, Tschopp A et al (2010) Influence of bowel preparation before 18F-FDG PET/CT on physiologic 18F-FDG activity in the intestine. J Nucl Med. https://doi.org/10.2967/jnumed.109.071001
Zhao L, Pang Y, Luo Z, Fu K, Yang T, Zhao L, Sun L, Wu H, Lin Q, Chen H. Role of [68Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with [18F]-FDG PET/CT Eur J Nucl Med Mol Imaging. 2021;48:1944–55
Kalluri R (2016) The biology and function of fibroblasts in cancer. NatRevCancer. https://doi.org/10.1038/nrc.2016.73
Scanlan MJ, Raj BK, Calvo B et al (1994) Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.91.12.5657
Loktev A, Lindner T, Burger EM et al (2019) Development of novel FAP-targeted radiotracers with improved tumor retention. J Nucl Med 60:1421–1429
Lindner T, Loktev A, Altmann A et al (2018) Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med 59:1415–1422
Röhrich M, Loktev A, Wefers AK et al (2019) IDH-wildtypeglioblastomas and grade III/IV IDH-mutant gliomas show elevated tracer uptake in fibroblast activation protein-specific PET/CT. Eur J Nucl Med Mol Imaging 46:2569–2580
Giesel FL, Heussel CP, Lindner T et al (2019) FAPI-PET/CT improves staging in a lung cancer patient with cerebral metastasis. Eur J Nucl Med Mol Imaging 46:1754–1755
Giesel FL, Kratochwil C, Lindner T et al (2019) 68Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAPI targeting agents in patients with various cancers. J Nucl Med 60:386–392
Loktev A, Lindner T, Mier W et al (2018) A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med 59:1423–1429
Kratochwil C, Flechsig P, Lindner T et al (2019) 8Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 60:801–805
Chen H, Pang Y, Wu J et al (2020) Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-020-04769-z
Chen H, Zhao L, Ruan D, Pang Y, Hao B, Dai Y et al (2020) Usefulness of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [(18)F]FDG PET/CT findings. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-020-04940-6
Pang Y, Zhao L, Luo Z, Hao B, Wu H, Lin Q, Sun L, Chen H (2021) Comparison of 68Ga-FAPI and 18F-FDG uptake in gastric, duodenal, and colorectal cancers. Radiology. https://doi.org/10.1148/radiol.2020203275
Kuten J, Levine C, Shamni O, Pelles S, Wolf I, Lahat G, Mishani E, Even-Sapir E (2021) Head-to-head comparison of [68Ga]Ga-FAPI-04 and [18F]-FDG PET/CT in evaluating the extent of disease in gastric adenocarcinoma. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-021-05494-x
Jiang D, Chen X, You Z, Wang H, Zhang X, Li X, Ren S, Huang Q, Hua F, Guan Y, Zhao J, Xie F (2021) Comparison of [68 Ga]Ga-FAPI-04 and [18F]-FDG for the detection of primary and metastatic lesions in patients with gastric cancer: a bicentric retrospective study. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-021-05441-w
Giesel FL, Kratochwil C, Schlittenhardt J, Dendl K, Eiber M, Staudinger F, Kessler L, Fendler WP, Lindner T, Koerber SA, Cardinale J, Sennung D, Roehrich M, Debus J, Sathekge M, Haberkorn U, Calais J, Serfling S, Buck AL (2021) Head-to-head intra-individual comparison of biodistribution and tumor uptake of 68Ga-FAPI and 18F-FDG PET/CT in cancer patients. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-021-05307-1
Şahin E, Elboğa U, Çelen YZ, Sever ÖN, Çayırlı YB, Çimen U (2021) Comparison of 68Ga-DOTA-FAPI and 18FDG PET/CT imaging modalities in the detection of liver metastases in patients with gastrointestinal system cancer. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2021.109867
Qin C, Shao F, Gai Y, Liu Q, Ruan W, Liu F, Hu F, Lan X (2021) 68Ga-DOTA-FAPI-04 PET/MR in the evaluation of gastric carcinomas: comparison with 18F-FDG PET/CT. J Nucl Med. https://doi.org/10.2967/jnumed.120.258467
Wang H, Zhu W, Ren S, Kong Y, Huang Q, Zhao J, Guan Y, Jia H, Chen J, Lu L, Xie F, Qin L (2021) 68Ga-FAPI-04 Versus 18F-FDG PET/CT in the detection of hepatocellular carcinoma. Front Oncol. https://doi.org/10.3389/fonc.2021.693640
Tabrizian P, Shrager B, Jibara G, Yang MJ, Romanoff A, Hiotis S, Sarpel U, Labow DM (2014) Cytoreductive surgery and hyperthermicintraperitoneal chemotherapy for peritoneal carcinomatosis: outcomes from a single tertiary institution. J Gastrointest Surg. https://doi.org/10.1007/s11605-014-2477-5
Dirisamer A, Schima W, Heinisch M, Weber M, Lehner HP, Haller J, Langsteger W (2009) Detection of histologically proven peritoneal carcinomatosis with fused 18F-FDG-PET/MDCT. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2007.11.032
Kim SJ, Lee SW. Diagnostic accuracy of 18F-FDG PET/CT for detection of peritoneal carcinomatosis: a systematic review and meta-analysis. Br J Radiol. 2018; https://doi.org/10.1259/bjr.20170519
Zhao L, Pang Y, Luo Z, Fu K, Yang T, Zhao L, Sun L, Wu H, Lin Q, Chen H (2021) Role of [68Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with [18F]-FDG PET/CT. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-020-05146-6
Lu YY, Chen JH, Ding HJ, Chien CR, Lin WY, Kao CH (2012) A systematic review and meta-analysis of pretherapeutic lymph node staging of colorectal cancer by 18FFDG PET or PET/CT. Nucl Med Commun 33(11):1127–1133
Coburn NG (2009) Lymph nodes and gastric cancer. J SurgOncol 99(4):199–206
Seevaratnam R, Cardoso R, McGregor C et al (2012) How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis Gastric Cancer 15(1):3–18
Findlay JM, Antonowicz S, Segaran A et al (2019) Routinely staging gastric cancer with 18F-FDG PET-CT detects additional metastases and predicts early recurrence and death after surgery. EurRadiol 29(5):2490–2498
Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, Wei J, Wu S, Zhao L, Luo Z, Lin X, Xie C, Sun L, Lin Q, Wu H (2020) Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-020-04769-z
Koerber SA, Staudinger F, Kratochwil C, Adeberg S, Haefner MF, Ungerechts G, Rathke H, Winter E, Lindner T, Syed M, Bhatti IA, Herfarth K, Choyke PL, Jaeger D, Haberkorn U, Debus J, Giesel FL (2020) The Role of 68Ga-FAPI PET/CT for patients with malignancies of the lower gastrointestinal tract: first clinical experience. J Nucl Med. https://doi.org/10.2967/jnumed.119.237016
Kinoshita T, Kinoshita T, Saiura A, Esaki M, Sakamoto H, Yamanaka T (2015) Multicentre analysis of long-term outcome after surgical resection for gastric cancer liver metastases. Br J Surg 102(1):102–107
Hashiguchi Y, Muro K, Saito Y et al (2020) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J ClinOncol 25(1):1–42
Giesel FL, Kratochwil C, Lindner T et al (2019) 68Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med 60(3):386–392
Guo W, Pang Y, Yao L, Zhao L, Fan C, Ke J, Guo P, Hao B, Fu H, Xie C, Lin Q, Wu H, Sun L, Chen H (2021) Imaging fibroblast activation protein in liver cancer: a single-center post hoc retrospective analysis to compare [68Ga]Ga-FAPI-04 PET/CT versus MRI and [18F]-FDG PET/CT. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-020-05095-0
Guadagni S, Catarci M, Kinoshita T, Valenti M, De Bernardinis G, Carboni M (1997) Causes of death and recurrence after surgery for early gastric cancer. World J Surg 21:434–439
Nakamura K, Tomioku M, Nabeshima K, Yasuda S (2014) Clinicopathologic features and clinical outcomes of gastric cancer patients with bone metastasis. Tokai J ExpClin Med 39:193–198
Yoshikawa K, Kitaoka H (1983) Bone metastasis of gastric cancer. Jpn J Surg 13:173–176
Turkoz FP, Solak M, Kilickap S et al (2014) Bone metastasis from gastric cancer: the incidence, clinicopathological features, and influence on survival. J Gastric Cancer 14:164–172
Hernandez RK et al (2018) Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. https://doi.org/10.1186/s12885-017-3922-0
Nozue M et al (2002) Treatment and prognosis in colorectal cancer patients with bone metastasis. Oncol Rep 9:109–112
Liu F et al (2016) Prognostic risk factors in patients with bone metastasis from colorectal cancer. Tumour Biol. https://doi.org/10.1007/s13277-016-5465-4
Author information
Authors and Affiliations
Contributions
Umut Elboga: conception of the work, revising work critically for important intellectual content, interpretation of data for the work. Ertan Sahin: design of the work, revising work critically for important intellectual content, interpretation of data for the work. Tulay Kus: analysis and interpretation of data for the work, revising work critically for important intellectual content. Yusuf Burak Cayirli: design of the work, interpretation of data for the work, drafting the work. Gokmen Aktas: interpretation of data for the work, revising work critically for important intellectual content. Merve Okuyan: acquisition and interpretation of data for the work, drafting the work. HavvaYesil Cinkir: acquisition of data for the work, revising work critically for important intellectual content. Fatih Teker: acquisition of data for the work, revising work critically for important intellectual content. Ozlem Nuray Sever: acquisition of data for the work, revising work critically for important intellectual content. Alper Aytekin: acquisition of data for the work, revising work critically for important intellectual content. Latif Yılmaz: acquisition of data for the work, revising work critically for important intellectual content. Aydın Aytekin: acquisition of data for the work, revising work critically for important intellectual content. Ufuk Cimen: acquisition of data for the work, drafting the work. Vuslat Mumcu: acquisition of data for the work, drafting the work. Benan Kilbas: interpretation of data for the work, revising work critically for important intellectual content. Kurtulus Eryilmaz: acquisition of data for the work, drafting the work. Davut Cakici: acquisition of data for the work, drafting the work. Yusuf Zeki Celen: interpretation of data for the work, revising work critically for important intellectual content.
All authors approve the final version of the work to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elboga, U., Sahin, E., Kus, T. et al. Comparison of 68Ga-FAPI PET/CT and 18FDG PET/CT Modalities in Gastrointestinal System Malignancies with Peritoneal Involvement. Mol Imaging Biol 24, 789–797 (2022). https://doi.org/10.1007/s11307-022-01729-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01729-x