Abstract
Introduction
Dysregulated activity of matrix metalloproteinases (MMPs) drives a variety of pathophysiological conditions. Non-invasive imaging of MMP activity in vivo promises diagnostic and prognostic value. However, current targeting strategies by small molecules are typically limited with respect to the bioavailability of the labeled MMP binders in vivo. To this end, we here introduce and compare three chemical modifications of a recently developed barbiturate-based radiotracer with respect to bioavailability and potential to image MMP activity in vivo.
Methods
Barbiturate-based MMP inhibitors with an identical targeting unit but varying hydrophilicity were synthesized, labeled with technetium-99m, and evaluated in vitro and in vivo. Biodistribution and radiotracer elimination were determined in C57/BL6 mice by serial SPECT imaging. MMP activity was imaged in a MMP-positive subcutaneous xenograft model of human K1 papillary thyroid tumors. In vivo data were validated by scintillation counting, autoradiography, and MMP immunohistochemistry.
Results
We prepared three new 99mTc‐labeled MMP inhibitors, bearing either a glycine ([99mTc]MEA39), lysine ([99mTc]MEA61), or the ligand HYNIC with the ionic co-ligand TPPTS ([99mTc]MEA223) yielding gradually increasing hydrophilicity. [99mTc]MEA39 and [99mTc]MEA61 were rapidly eliminated via hepatobiliary pathways. In contrast, [99mTc]MEA223 showed delayed in vivo clearance and primary renal elimination. In a thyroid tumor xenograft model, only [99mTc]MEA223 exhibited a high tumor-to-blood ratio that could easily be delineated in SPECT images.
Conclusion
Introduction of HYNIC/TPPTS into the barbiturate lead structure ([99mTc]MEA223) results in delayed renal elimination and allows non-invasive MMP imaging with high signal-to-noise ratios in a papillary thyroid tumor xenograft model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Matrix metalloproteinases (MMPs) comprise a subfamily of the metzincins and belong to the zinc‐ and calcium depending endopeptidases. MMPs are involved in a variety of physiological processes but also play a crucial role in different pathophysiological conditions, e.g., in cancer, joint disorders (including rheumatoid arthritis and osteoarthritis), neurodegenerative diseases, respiratory disorders, cardiovascular disease, and many more [1].
MMPs are capable to enzymatically cleave the protein components of the extracellular matrix (ECM) with overlapping substrate specificities. Moreover, MMPs are involved in processing bioactive molecules such as proteinase inhibitors, growth factors, cytokines, and chemokines [2, 3].
The non-invasive detection and assessment of locally upregulated and activated matrix metalloproteinases (MMPs) in vivo using MMP inhibitor-based radiotracers for positron emission tomography (PET) or single photon emission computed tomography (SPECT) is still a challenge [4]. However, if successful, the visualization of MMP activity by means of aforementioned scintigraphic technologies would become a breakthrough by improving diagnosis and assessment of disease progression [5].
Several groups are working on the design, improvement, and evaluation of MMP inhibitor-based radiotracers aiming at the non-invasive imaging of MMP-associated diseases by means of SPECT or PET [4, 6,7,8,9,10,11,12]. For this purpose, different classes of radiolabeled MMP inhibitors (MMPIs) have been developed and explored as radiotracers. Radiolabeled hydroxamate-based MMPIs have been successfully applied to image MMP activity, for example in preclinical models of atherosclerosis [13, 14] and stroke [15] and to visualize MMP activity in patients with multiple sclerosis [16]. These lead structures mainly behave like so called combined or right-hand side MMP inhibitors depending on the substituents occupying both the S1‐S3 and S1’‐S3’ enzyme pockets (combined) or S1’‐S3’ enzyme pockets (right hand side). Via such MMP inhibitor radiotracer approaches, it is putatively feasible to follow locally upregulated MMPs in their activated forms in vivo [5, 13, 17,18,19,20]. Disadvantages of hydroxamates in MMP imaging are their broad inhibition spectrum, metabolic instability, as well as interactions with other metalloproteinases due to their high transition-metal binding potential [21]. Non-hydroxamate-based MMPIs like substituted pyrimidine-2,4,6-triones (barbiturates) often possess higher specificity for the gelatinases MMP-2 and MMP-9 [22] and were the basis for the development of C-5-disubstituted barbiturates with improved MMP specificity and potency [23]. We have introduced 18F-labeled C5‐disubstituted barbiturates as potential MMP‐targeted radiotracers, putatively binding to the zinc ion at the active site via the enolic tautomer of the barbiturate moiety [24,25,26]. Moreover, we have also presented a first 68Ga‐labeled version of a barbiturate which was synthesized by azide‐alkyne cycloaddition. This potential PET tracer was the first radiometal-labeled MMP inhibitor based on a barbiturate lead structure reported so far [27]. All these examples are characterized by fast blood clearance and therefore short availability for binding to the target enzymes which is mainly caused by their pharmacokinetic profile rather than by their binding properties to the active MMPs. Introduction of ionic charges like in [99mTc]RP805, a cyclic hydroxamate-based MMP-radiotracer, gave promising results in imaging of MMP activation [28].
Therefore, we developed and evaluated three MMPIs presenting a novel series of 99mTc‐labeled barbiturates with gradually increasing hydrophilicity. Starting with a glycine-spacer ([99mTc]MEA39), we increased hydrophilicity by changing the spacer to lysine ([99mTc]MEA61) and finally introduced HYNIC as a bifunctional coupling agent for 99mTc-labeling, together with TPPTS and tricine as co-ligands, resulting in high hydrophilicity with regard to the final radiotracer ([99mTc]MEA223). We evaluated the impact of altered MMPI hydrophilicity on tracer dynamics and clearance in wild-type mice in vivo. Furthermore, we investigated the potential of these 99mTc‐labeled barbiturates to non-invasively assess tumor-associated MMP activity, specifically whether the altered tracer hydrophilicity and dynamics leads to improved tumor/blood contrast.
Materials and Methods
Chemistry
All chemicals, reagents, and solvents for the syntheses of the compounds were analytical grade, purchased from commercial sources and used without further purification unless otherwise specified. For radiosynthesis, only solvents of pharmaceutical purity (Reag.Ph.Eur.) were used.
The labeling of compounds was accomplished by using two different kits which both are prepared in house. Briefly, one labeling kit to produce a triaquatricarbonyl-complex [99mTc(CO)3(OH2)3]+ was used for radiosynthesis of [99mTc]MEA39 and [99mTc]MEA61; the second one was used for the HYNIC-derivative [99mTc]MEA223 (see SI). The precursors and corresponding kits were heated to 100 °C for 16–20 min. After cooling to room temperature, the crude reaction mixtures were purified by HPLC. Products were collected, diluted with water, and filtered through Sep-Pak® C-18 Plus cartridges. Solvents were removed under reduced pressure without heating, and the residues were solved in 0.9% NaCl-solution containing 1.6 vol% of Tween80® (100–500 µL). For quality control, all injectable solutions were analyzed determining the radiochemical purity (RCP), the pH-value, and osmolality.
Identification of labeled compounds was performed by co-injection of a non-radioactive Rhenium-based reference on HPLC. In the case of [99mTc]MEA223, a reference compound could not be synthesized, and the identification of the corresponding 99gTc-derivative (after decay) was done by mass spectrometry.
The serum stability of all radiolabeled compounds was evaluated by incubation in human and murine serum at 37 °C for up to 120 min and analyzed by HPLC. The distribution coefficients (logDexp) were determined in a two-phase system consisting of 1-octanol and PBS-buffer (pH = 7.4) according to the literature [29].
Animals
All animal experiments performed in the study were in accordance with the German Law on the Care and Use of Laboratory Animals and approved by the local authorizing agency of North Rhine-Westphalia.
C57/BL6 mice (female, 12–15 weeks, 20–23 g) were anesthetized with 2% isoflurane (Abbott Animal Health) in 100% O2, and a lateral tail vein catheter was placed using a 27G needle connected to 15-cm polyethylene tubing. 80–100 MBq of the respective tracer was injected as a bolus (100 μL compound flushed with 100 μL saline) via the tail vein, and subsequent SPECT imaging was performed.
For tumor studies, 2 × 106 K1-LITG human thyroid cancer cells in 40–60 µl plain DMEM medium (Thermo Fisher Scientific, Waltham, USA) were subcutaneously injected above each shoulder of CD1nude/nude mice (Charles River, female, 9–10 weeks, 25–28 g). Imaging experiments were performed 15 days post-implantation. All animals were randomly assigned to experimental groups.
SPECT/CT Imaging
SPECT experiments were carried out using a small-animal SPECT/CT scanner (NanoScan, Mediso). For biodistribution studies, dynamic SPECT scans were acquired over the course of 90 min p.i. (9 × 10 min frames, field of view 108 mm). Following the acquisition, CT contrast agent (Ultravist®-370, 5 µl/g bw) was injected via the tail vein catheter, and a CT image was obtained. Mice underwent subsequent SPECT/CT scans 4 h p.i. (1 × 30 min frame) and 24 h p.i. (1 × 60 min frame). For in vivo tumor uptake studies, mice were imaged 0–60 min and 4 h p.i. of tracer with a reduced field of view (1 × 30 min frame, 26 mm).
Reconstructed image data sets were analyzed using the in-house developed software MEDgical. Three-dimensional volumes of interest (VOIs) were defined over the respective organs in CT data sets, transferred to the co-registered SPECT data, and analyzed quantitatively. Regional uptake was calculated as percentage injected dose (%ID) or standardized uptake units (SUV) by decay-correcting all data to the time of injection and subsequently dividing counts per milliliter in the VOI by total injected dose and normalizing to bodyweight. Total elimination was analyzed by thresholding the abdominal region in dynamic SPECT acquisitions. Renal elimination was defined as the sum of radioactivity in kidneys and urinary bladder, hepatobiliary elimination as the difference between total elimination and renal elimination.
Ex Vivo Validation
Following the last SPECT/CT acquisition, mice were euthanized by cervical dislocation and a necropsy was performed. Ex vivo biodistribution of radioactivity was analyzed by scintillation counting (Wizard2 gamma counter, Perkin Elmer), and the radioactivity in respective organs was decay-corrected and calculated as %ID per Gram tissue (% ID/g).
Directly after scintillation counting, tumors were embedded and snap-frozen in Tissue-Tek (Tissue-Tek OCT Weckert Labortechnik, Kitzingen, Germany). For autoradiography, 20-µm frozen tissue sections were measured for 6 h in a microimager (Biospace Lab, Nesles la Vallee, France). Adjacent Sects. (10 µm) were collected for histological analysis and stained with an anti-MMP-9 antibody (abcam ab38889, 1:200, overnight at 4 °C), the appropriate secondary antibody (anti-rabbit A-21206, Invitrogen, 1:800) and DAPI (Vectashield, H-1500, Vector Laboratories, USA).
Statistical Analysis
Statistical significance was analyzed using 1-way or 2-way ANOVA and Tukey post-tests. P values of *p < 0.05, **p < 0.01, and ***p < 0.001 were considered significant. In-text values are mentioned as mean ± standard deviation.
Results
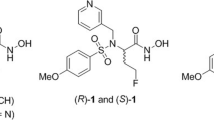
The synthesis of the three different 99mTc-labeled MMPIs starts with the preparation of the barbiturate 11 with an azido PEG-chain for installing the different Tc-chelators in all cases [30]. This azide-functionalized derivative was also characterized by crystal structure which is outlined together with the synthetic procedures in the supporting information. For the first two 99mTc-labeled tracers [99mTc]MEA39 and [99mTc]MEA61, we applied the “click to chelate” concept [31]. In contrast to the literature, the corresponding precursors for labeling which are usually prepared in situ were isolated because direct labeling failed. We assume that this is likely being caused by the two very basic tertiary amines of the piperazine ring interfering with the amino acid. This makes an in situ formation of the necessary precursors impossible or at least unfavorable. For [99mTc]MEA39, the azide 11 was used in a click reaction with the commercially available fully functionalized (S)-2-[(tert-butoxycarbonyl)amino]pent-4-ynoic acid to get the protected precursor 13. After deprotection and purification (as potassium salt), the corresponding Re-complex MEA39 was prepared as cold reference using [NEt4]2[ReBr3(CO)3][32] (Scheme 1). For radiolabeling an in-house prepared [99mTc(CO)3(OH2)3]+-kit was used yielding [99mTc]MEA39 with a radiochemical yield of 21 ± 11% (n = 6) and reproducible radiochemical purity of over 99%. The total time for synthesis was 131 min ± 9 min (n = 6). The identity of the product was verified by HPLC by co-injection of the analogous Rhenium-complex MEA39 as well as by mass spectrometry. For [99mTc]MEA61, we installed the more polar lysine-derivative 8 which was prepared according to the literature [33]. After forming the triazole and subsequent deprotection, the resulting precursor 17 was converted into the corresponding rhenium complex (MEA61) and radiolabeled with technetium-99 m ([99mTc]MEA61) in the same way as mentioned before. The radiochemical yield was comparable with 23 ± 11% (n = 3) and radiochemical purities of over 99% (130 min total synthesis time).
Synthesis and radiolabeling of [99mTc]MEA39 and [99mTc]MEA61: (a) (S)-2-[(tert-butoxycarbonyl)amino]pent-4-ynoic acid, CuSO4 · 5H2O, sodium ascorbate, DMF, H2O, rt, 12 h, 40%; (b) (I) TFA, CH2Cl2, rt, 18 h, (II) K2CO3, THF, rt, 5 h, 16% (two steps); (c) [NEt4]2[ReBr3(CO)3], MeOH, H2O, 70 °C, 5 h, then rt, 18 h, 18%; (d) [99mTc(CO)3(OH2)3]+, 100 °C, 16 min., rcy 21% d.c., rcp > 99% (e) 8, CuSO4 · 5H2O, Na ascorbate, DMF, H2O, rt, 12 h, 48%; (f) (I) TFA, CH2Cl2, rt, 18 h, 63% (II) K2CO3, THF, rt, 5 h, 79%; (g) [NEt4]2[ReBr3(CO)3], MeOH, H2O, 70 °C, 5 h, then rt, 18 h, 41%; (h) [99mTc(CO)3(OH2)3]+, 100 °C, 16 min., rcy 23% d.c., rcp > 99%. (d.c.: decay corrected; rcy: radiochemical yield; rcp: radiochemical purity).
In order to further improve solubility and hydrophilicity of the envisioned tracers, a HYNIC-functionalized barbiturate was prepared. After Staudinger-reduction of the azide 11 to the free amine, the active ester 2,5-dioxopyrrolidin-1-yl-6-[2-(tert-butoxycarbonyl)-hydrazinyl]nicotinate was used to form the amide 18. The Boc-group was removed with hydrochloric acid in dioxane yielding the precursor 19. For radiolabeling with technetium-99 m, suitable co-ligands are necessary to get tracers of reasonable stability. To reach high hydrophilicity, we choose tricine and triphenylphosphine trisulfonate (TPPTS) as co-ligands and synthesized [99mTc]MEA223 with a radiochemical yield of 34 ± 10% (n = 10) and reproducible radiochemical purity of over 99% via standard kit-procedure (Scheme 2) [34]. The total time for synthesis was 143 min ± 30 min (n = 10). Under these conditions, the synthesis of the corresponding rhenium complex failed. It is known that rhenium-labeled HYNIC-biomolecule conjugates are often lacking stability [35, 36]. Therefore we performed mass spectrometry to verify [99gTc]MEA223 (see SI).
Synthesis and radiolabeling of [99mTc]MEA223: (a) PPh3, THF, H2O, rt, 2d, 58%; (b) 2,5-Dioxopyrrolidin-1-yl-6-[2-(tert-butoxycarbonyl)hydrazinyl]nicotinate, THF, NEt3, rt, 12 h, 79%; (c) HCl, dioxane, MeOH, THF, 4d, 18% (d) [99mTcO4]−, 3,3′,3″-phosphanetriyltris-(benzenesulfonic acid) trisodium salt (TPPTS), tricine, mannitol, disodium succinate hexahydrate, succinic acid, 100 °C, 20 min., rcy 34% d.c., rcp > 99%. (d.c.: decay corrected; rcy: radiochemical yield; rcp: radiochemical purity).
In vitro Characterization
In order to evaluate the affinity of the new barbiturate-based compounds towards MMPs, an in vitro inhibition study was performed. The IC50-values were determined for MMP-2, MMP-8, MMP-9, MMP-13, and MMP-15 using the protocol from Huang et al. [37] The rhenium-labeled non-radioactive counterparts MEA39 and MEA61 were used in this assay, and the results are summarized in Table 1. Additionally, the corresponding precursors were also measured and outlined in the SI for comparison. For [99mTc]MEA223 the determination of IC50-values was not possible because of lacking the Re-labeled non-radioactive derivative (see above).
The amino acid-based barbiturates MEA39 and MEA61 show high affinity towards the tested MMPs in the nanomolar range. The glycine-based MEA39 has some specificity towards the gelatinases MMP-2 and MMP-9 over MMP-8, while the lysine-based MEA61 shows selectivity for the gelatinases over MMP-13 and MMP-14.
Experimental logD value were determined [29] and showed decreasing lipophilicity from [99mTc]MEA39 (0.79 ± 0.03) to [99mTc]MEA61 (0.35 ± 0.23) and [99mTc]MEA223 (0.15 ± 0.07). in vitro stability tests were performed by incubating tracers in human and murine blood serum followed by HPLC analysis for 2 h. All tracers proved to be stable with no detectable radiometabolites or decomposition products in both human and murine serum after 120 min. (see SI).
Preclinical Evaluation
Biodistribution
In vivo biodistribution was determined in adult female C57/BL6 mice after intravenous injection. Representative whole-body images (maximum intensity projections) of investigated radiotracers at 0–10, 20–30, 50–60, and 80–90 min post injection (p.i.) are shown in Fig. 1A.
In vivo distribution and excretion analysis of barbiturates in adult C57/Bl6 mice after intravenous injection. A Maximum intensity projections of the biodistribution of radiotracers [99mTc]MEA39, [99mTc]MEA61, and [99mTc]MEA223 at increasing time points post injection. B Dynamic in vivo blood radioactivity determined from left ventricular volume of interest (VOI). C in vivo radiotracer accumulation in the liver. D Radioactivity accumulation (expressed as percentage of injected dose) in excretion organs (liver, gallbladder, intestine, kidney, bladder) at 10 and 90 min p.i. and relative percentage of hepatobiliary and renal elimination pathways. Data are shown as mean ± SD (n = 4–5), ID, injected dose; image orientation L, left. Statistical significance was calculated using 2-way ANOVA and Tukey post-test, and stars indicate significance of differences of radiotracer uptake of [99mTc]MEA223 compared to the other compounds: *p < 0.05, **p < 0.01, ***p < 0.001.
[99mTc]MEA39 presented with a fast clearance from the blood with 91.5 ± 3.2% of the injected dose (%ID) accumulating in the excretion organs liver and kidney already within the first 10 min (Fig. 1B, C). After 90 min p.i., 96.4 ± 2.0%ID were excreted, the vast majority via the hepatobiliary system (Fig. 1D). Elimination of [99mTc]MEA61 was equally fast, showing 93.0 ± 3.6%ID accumulating in excretion organs after 90 min p.i.. Similar to [99mTc]MEA39, the majority of [99mTc]MEA61 (92.0%) was eliminated via the hepatobiliary pathway.
In contrast, [99mTc]MEA223 showed a delayed elimination with only 66.6 ± 4.4%ID found in excretion organs 90 min p.i.. First pass effect and early accumulation in the liver was significantly reduced ([%ID/ml] at 10 min p.i.: 7.3 ± 0.5 vs. 38.1 ± 9.3 ([99mTc]MEA61) vs. 50.8 ± 9.3 ([99mTc]MEA39) (Fig. 1C), and elimination was shifted towards renal elimination (64.4 ± 9.3%). Until 60 min p.i., [99mTc]MEA223 showed significantly higher radioactivity in the blood than [99mTc]MEA39 and [99mTc]MEA61 (Fig. 1B).
Additionally, late time point acquisitions were performed 4 h and 24 h after tracer injection to assess delayed kinetics. Representative examples are shown in Fig. 2. Four hours p.i. blood radioactivity of [99mTc]MEA223 and [99mTc]MEA61 remained considerably high ([%ID/ml]: 0.8 ± 0.6 and 0.8 ± 0.5), while [99mTc]MEA39 showed an increased wash out and lower blood concentrations ([%ID/ml]: 0.2 ± 0.1) as shown in Fig. 2B and C. While the blood radioactivity of [99mTc]MEA223 decreased further over the next 20 h ([%ID/ml] at 24 h p.i.: 0.1 ± 0.1), [99mTc]MEA61 presented with a long circulation time and unchanged blood radioactivity ([%ID/ml] at 24 h p.i.: 1.0 ± 0.7). Radiotracer accumulation in non-excretion organs 4 and 24 h p.i. was very low for all compounds with < 1% ID/ml, but, due to increased circulation times, as a tendency slightly higher for [99mTc]MEA61. Complementary ex vivo measurements of tissue samples at 25 h p.i. confirmed the in vivo data at the latest time point (24 h p.i.) as shown in Fig. 2D.
Late time point in vivo biodistribution of barbiturates in adult C57/Bl6 mice. Maximum intensity projections A and quantification B, C of the biodistribution of radiotracers [99mTc]MEA39, [99mTc]MEA61, and [99mTc]MEA223 4 and 24 h p.i. D Ex vivo biodistribution determined by scintillation counting 25 h p.i. Data shown as box plot min to max (n = 5–6). Statistical significance was calculated using 2-way ANOVA and Tukey post-test: *p < 0.05, **p < 0.01, ***p < 0.001.
In vivo Imaging of Tumor MMP Activity
We applied the three radiotracers for in vivo imaging of MMP activity in a subcutaneous xenograft model of human K1 papillary thyroid tumors which are known for high MMP activity. MMP-2 and MMP-9 activity in patients with papillary thyroid tumors are linked to tumor cell invasion and metastasis, and high gelatinase activity has been associated with poor prognosis [38, 39].
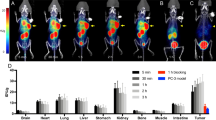
Representative SPECT images (axial sections) of radiotracer uptake at 4 h p.i. coregistered to a CT are shown in Fig. 3A.
In vivo imaging of MMP activity in a subcutaneous xenograft tumor model. A Axial SPECT images coregistered to CT showing in vivo tumor signals 4 h post radiotracer injection (error heads point at s.c. tumors). B, C Quantification of in vivo tumor signals and tumor-to- blood ratios 1 h and 4 h p.i., respectively. D Ex vivo tumor signals determined by scintillation counting and tumor-to-blood ratios 5 h p.i. Data shown as mean ± SD (n = 5). Image orientation L, left; SUV, standardized uptake units; %ID/g, % injected dose per gram. Statistical significance was calculated using 1-way ANOVA and Tukey post-tests: *p < 0.05, **p < 0.01, ***p < 0.001.
1 h p.i., in vivo tumor signal was highest for [99mTc]MEA223, followed by [99mTc]MEA61 and [99mTc]MEA39. Differences in early tumor signals were in accordance with differences in blood radioactivity, as tumor-to-blood ratios remained below 1 for all radiotracers (Fig. 3B). 4 h p.i., [99mTc]MEA223 showed a strong accumulation in tumors, independent of local tumor perfusion with positive tumor-to-blood ratios. In contrast, [99mTc]MEA61 and [99mTc]MEA39 showed no specific tumor accumulation, as tumor signals declined parallel to the decrease in blood radioactivity ([tumor/blood in vivo 4 h p.i.]: 1.54 ± 0.19 ([99mTc]MEA223) vs. 0.47 ± 0.08 ([99mTc]MEA61) vs. 0.46 ± 0.14 ([99mTc]MEA39) (Fig. 3C). In vivo results could be confirmed by ex vivo scintillation counting ([tumor/blood ex vivo 5 h p.i.]: 7.12 ± 3.37 ([99mTc]MEA223) vs. 0.44 ± 0.19 ([99mTc]MEA61) vs. 0.49 ± 0.22 ([99mTc]MEA39) (Fig. 3D). Additionally, ex vivo autoradiography confirmed that only [99mTc]MEA223 showed relevant radiotracer accumulation throughout the tumor, even though all tumors were highly positive for the target in MMP immunohistochemistry (Fig. 4).
Conclusion
We successfully prepared two new [99mTc]Tc(CO)3‐labeled MMP inhibitors, bearing either a glycine or a lysine residue within the 99mTc‐chelator. In a third compound, HYNIC was used together with TPPTS and tricine as co-ligands. All tracers were prepared with high radiochemical purities and reproducible radiochemical yields. In vitro experiments showed high stability of all tracers in human and murine blood serum, and excellent affinity of the amino acid-based reference compounds MEA39 and MEA61 towards targeted matrix metalloproteinases. As expected, HYNIC-based [99mTc]MEA223 showed increased hydrophilicity, as compared to [99mTc]MEA39 and [99mTc]MEA61. In vivo, amino acid-based tracers [99mTc]MEA39 and [99mTc]MEA61 were rapidly eliminated via hepatobiliary pathways. In contrast, the more hydrophilic [99mTc]MEA223 was primarily excreted via the kidneys and showed a significantly increased bioavailability for the first 90 min after injection. We demonstrated the relevance and impact of the altered tracer kinetics in a thyroid tumor xenograft model. Here, [99mTc]MEA223 exhibited a high tumor-to-blood ratio that could easily be delineated in SPECT images. The newly developed [99mTc]MEA223 hence allows non-invasive imaging of MMP activity with high signal-to-noise and should be investigated in additional pathophysiological conditions.
References
Gomis-Rüth FX, Botelho TO, Bode W (2012) A standard orientation for metallopeptidases. Biochim Biophys Acta Proteins Proteom BBA-PROTEINS PROTEOM 1824:157–163. https://doi.org/10.1016/j.bbapap.2011.04.014
Fanjul-Fernández M, Folgueras AR, Cabrera S, López-Otín C (2010) Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta. Mol Cell Res 1803:3–19. https://doi.org/10.1016/j.bbamcr.2009.07.004
Rodríguez D, Morrison CJ, Overall CM (2010) Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim. Biophys. Acta. Mol Cell Res 1803:39–54. https://doi.org/10.1016/j.bbamcr.2009.09.015
Matusiak N, Waarde A, Bischoff R et al (2013) Probes for non-invasive matrix metalloproteinase-targeted imaging with PET and SPECT. Curr Pharm Des 19:4647–4672. https://doi.org/10.2174/1381612811319250011
Wagner S, Breyholz H-JJ, Faust A et al (2006) Molecular imaging of matrix metalloproteinases in vivo using small molecule inhibitors for SPECT and PET. Curr Med Chem 13:2819–2838. https://doi.org/10.2174/092986706778522002
Auf Dem Keller U, Bellac CL, Li Y et al (2010) Novel matrix metalloproteinase inhibitor [18F]marimastat-aryltrifluoroborate as a probe for in vivo positron emission tomography imaging in cancer. Cancer Res. 70:7562–7569. https://doi.org/10.1158/0008-5472.CAN-10-1584
Casalini F, Fugazza L, Esposito G et al (2013) Synthesis and preliminary evaluation in tumor bearing mice of new 18F-labeled arylsulfone matrix metalloproteinase inhibitors as tracers for positron emission tomography. J Med Chem 56:2676–2689. https://doi.org/10.1021/jm4001743
Razavian M, Tavakoli S, Zhang J et al (2011) Atherosclerosis plaque heterogeneity and response to therapy detected by in vivo molecular imaging of matrix metalloproteinase activation. J Nucl Med 52:1795–1802. https://doi.org/10.2967/jnumed.111.092379
Razavian M, Nie L, Challa A et al (2014) Lipid lowering and imaging protease activation in atherosclerosis. J Nucl Cardiol 21:319–328. https://doi.org/10.1007/s12350-013-9843-7
Hugenberg V, Riemann B, Hermann S et al (2013) Inverse 1,2,3-triazole-1-yl-ethyl substituted hydroxamates as highly potent matrix metalloproteinase inhibitors: (radio)synthesis, in vitro and first in vivo evaluation. J Med Chem 56:6858–6870. https://doi.org/10.1021/jm4006753
Ujula T, Huttunen M, Luoto P et al (2010) Matrix metalloproteinase 9 targeting peptides: syntheses, 68Ga-labeling, and preliminary evaluation in a rat melanoma xenograft model. Bioconjug Chem 21:1612–1621. https://doi.org/10.1021/bc1000643
Altıparmak B, Lambrecht FY, Citak A (2014) Design of radiolabeled gelatinase inhibitor peptide (99mTc-CLP) and evaluation in rats. Appl Radiat Isot 89:130–133. https://doi.org/10.1016/J.APRADISO.2014.02.012
Schäfers M, Riemann B, Kopka K et al (2004) Scintigraphic imaging of matrix metalloproteinase activity in the arterial wall in vivo. Circulation 109:2554–2559. https://doi.org/10.1161/01.CIR.0000129088.49276.83
Ohshima S, Petrov A, Fujimoto S et al (2009) Molecular imaging of matrix metalloproteinase expression in atherosclerotic plaques of mice deficient in apolipoprotein E or low-density-lipoprotein receptor. J Nucl Med 50:612–617. https://doi.org/10.2967/jnumed.108.055889
Zinnhardt B, Viel T, Wachsmuth L et al (2015) Multimodal imaging reveals temporal and spatial microglia and matrix metalloproteinase activity after experimental stroke. J Cereb Blood Flow Metab 278850:1–11. https://doi.org/10.1038/jcbfm.2015.149
Gerwien H, Hermann S, Zhang X, et al (2016) Imaging matrix metalloproteinase activity in multiple sclerosis as a specific marker of leukocyte penetration of the blood-brain barrier. Sci Transl Med 8:364ra152–364ra152. https://doi.org/10.1126/scitranslmed.aaf8020
Puerta DT, Lewis JA, Cohen SM (2004) New beginnings for matrix metalloproteinase inhibitors: identification of high-affinity zinc-binding groups. JACS 126:8388–8389. https://doi.org/10.1021/ja0485513
Kopka K, Breyholz H-JJ, Wagner S et al (2004) Synthesis and preliminary biological evaluation of new radioiodinated MMP inhibitors for imaging MMP activity in vivo. Nucl Med Bio 31:257–267. https://doi.org/10.1016/j.nucmedbio.2003.08.003
Hartung D, Schäfers M, Fujimoto S et al (2007) Targeting of matrix metalloproteinase activation for noninvasive detection of vulnerable atherosclerotic lesions. EJNMMI 34:1–8. https://doi.org/10.1007/s00259-007-0435-0
Georgiadis D, Yiotakis A (2008) Specific targeting of metzincin family members with small-molecule inhibitors: progress toward a multifarious challenge. Bioorg Med Chem 16:8781–8794. https://doi.org/10.1016/j.bmc.2008.08.058
Fisher JF, Mobashery S (2006) Recent advances in MMP inhibitor design. Cancer Metastasis Rev 25:115–136. https://doi.org/10.1007/s10555-006-7894-9
Grams F, Brandstetter H, D’Alò S et al (2001) Pyrimidine-2,4,6-Triones: a new effective and selective class of matrix metalloproteinase inhibitors. Biol Chem 382:1277–1285. https://doi.org/10.1515/BC.2001.159
Breyholz H-J, Schäfers M, Wagner S et al (2005) C-5-disubstituted barbiturates as potential molecular probes for noninvasive matrix metalloproteinase imaging. J Med Chem 48:3400–3409. https://doi.org/10.1021/jm049145x
Breyholz H-J, Wagner S, Faust A et al (2010) Radiofluorinated pyrimidine-2,4,6-triones as molecular probes for noninvasive MMP-targeted imaging. ChemMedChem 5:777–789. https://doi.org/10.1002/cmdc.201000013
Tochowicz A, Maskos K, Huber R et al (2007) Crystal structures of MMP-9 complexes with five inhibitors: contribution of the flexible Arg424 side-chain to selectivity. J Mol Biol 371:989–1006. https://doi.org/10.1016/j.jmb.2007.05.068
Brandstetter H, Grams F, Glitz D et al (2001) The 1.8-Å crystal structure of a matrix metalloproteinase 8-barbiturate inhibitor complex reveals a previously unobserved mechanism for collagenase substrate recognition. J Biol Chem 276:17405–17412. https://doi.org/10.1074/jbc.M007475200
Claesener M, Schober O, Wagner S, Kopka K (2012) Radiosynthesis of a 68Ga-labeled matrix metalloproteinase inhibitor as a potential probe for PET imaging. Appl Radiat Isot 70:1723–1728. https://doi.org/10.1016/J.APRADISO.2012.04.013
Su H, Spinale FG, Dobrucki LW et al (2005) Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation 112:3157–3167. https://doi.org/10.1161/CIRCULATIONAHA.105.583021
Prante O, Hocke C, Löber S et al (2006) Tissue distribution of radioiodinated FAUC113 Assessment of a pyrazolo(1,5-a) pyridine based dopamine D4 receptor radioligand candidate. Nuklearmedizin 45:41–48
Faust A, Waschkau B, Waldeck J et al (2008) Synthesis and evaluation of a novel fluorescent photoprobe for imaging matrix metalloproteinases. Bioconjug Chem 19:1001–1008. https://doi.org/10.1021/bc700409j
Mindt TL, Struthers H, Brans L et al (2006) “Click to chelate”: synthesis and installation of metal chelates into biomolecules in a single step. JACS 128:15096–15097. https://doi.org/10.1021/ja066779f
Alberto R, Egli A, Abram U, et al (1994) Synthesis and reactivity of [NEt4]2[ReBr3(CO)3]. Formation and structural characterization of the clusters [NEt4][Re3(µ3-OH)(µ-OH)3(CO)9] and [NEt4][Re2(µ-OH)3(CO)6] by alkaline titration. J Chem Soc Dalton Trans 0:2815–2820.https://doi.org/10.1039/DT9940002815
Mindt TL, Struthers H, Spingler B et al (2010) Molecular assembly of multifunctional 99mTc-radiopharmaceuticals using “Clickable” amino acid derivatives. ChemMedChem 5:2026–2038. https://doi.org/10.1002/cmdc.201000342
Ji S, Czerwinski A, Zhou Y et al (2013) 99mTc-Galacto-RGD2: a novel 99mTc-labeled cyclic RGD peptide dimer useful for tumor imaging. Mol Pharm 10:3304–3314. https://doi.org/10.1021/mp400085d
Melendez L, Decristoforo C, Mather SJ (2001) 53. Rhenium-188 labelling of HYNIC-octreotide analogues. Nucl Med Commun 22:452–452
Blower P (2017) Rhenium-188 radiochemistry: challenges and prospects. Int. J. Nucl. https://doi.org/10.15379/2408-9788.2017.04
Huang W, Meng Q, Suzuki K et al (1997) Mutational study of the amino-terminal domain of human tissue inhibitor of metalloproteinases 1 (TIMP-1) locates an inhibitory region for matrix metalloproteinases. J Biol Chem 272:22086–22091. https://doi.org/10.1074/jbc.272.35.22086
Marečko I, Cvejić D, Šelemetjev S et al (2014) Enhanced activation of matrix metalloproteinase-9 correlates with the degree of papillary thyroid carcinoma infiltration. Croat Med J 55:128–137. https://doi.org/10.3325/cmj.2014.55.128
Tian X, Cong M, Zhou W et al (2008) Relationship between protein expression of VEGF-C, MMP-2 and lymph node metastasis in papillary thyroid cancer. Int J Nucl 36:699–703. https://doi.org/10.1177/147323000803600411
Acknowledgements
The authors would like to thank Claudia Essmann, Renato Margeta, Christine Bätza, Sarah Köster, Stefanie Bouma, Roman Priebe, Christa Möllmann, and Irmgard Hoppe for the technical support. Furthermore, we like to thank David Clases and Uwe Karst for mass spectrometry of the 99gTc-compounds. This work was supported by funding from the Deutsche Forschungsgemeinschaft (DFG) — CRC/TR128 (project B03) & CRC 1450 — 431460824 (projects C01 & C03) and the IZKF Münster, Germany, core unit PIX.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Honold, Lisa and Austrup, Melanie contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Supporting information is provided including detailed procedures for all synthetic steps, analytical data and spectra copies of all new compounds, western blot data, as well as detailed information about radiosynthesis and animal experiments. CCDC 2,090,342 for compound 11 is contained in the supplementary crystallographic data for this manuscript. This data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. (DOCX 4084 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Honold, L., Austrup, M., Faust, A. et al. Towards Optimized Bioavailability of 99mTc-Labeled Barbiturates for Non-invasive Imaging of Matrix Metalloproteinase Activity. Mol Imaging Biol 24, 434–443 (2022). https://doi.org/10.1007/s11307-021-01668-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01668-z