Abstract
Objective
To investigate the dynamic change of amide proton transfer (APT) imaging before and after irradiation in nasopharyngeal carcinoma (NPC) and the underlying histopathological mechanism.
Materials and Methods
Tumor-bearing BALB/C nude mouse models were established and randomly divided into three groups: high-dose group (20 Gy/2 fractions), low-dose group (10 Gy/2 fractions), and control group (0 Gy). MRI scanning was performed before irradiation and 3rd, 6th, and 9th day post-irradiation. Scanning sequence included T1 weighted, T2 weighted, and APT. HE staining and TUNEL immunofluorescence detection were performed to detect necrosis and apoptosis.
Results
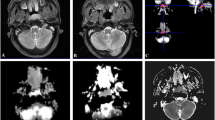
After high-dose irradiation, the mean tumor APT values decreased significantly on the 3rd day and 6th day (from 3.83 before radiotherapy to 2.41%, P < 0.001, 3rd day; from 2.41 to 1.80%, P = 0.001, 6th day). For low-dose irradiation, the mean tumor APT values decreased slightly on the 3rd day and 6th day (from 3.52 to 3.13%, P = 0.109, 3rd day; from 3.13 to 3.05%, P = 0.64, 6th day). The mean APT values of nonirradiated tumor changed slightly. In contrast, the average volume of high-dose irradiated tumors did not decrease obviously until the 9th day post-irradiation (from 290 before radiotherapy to 208 mm3 on the 9th day). The low-dose irradiated tumors showed slow growth, and the nonirradiated tumors showed rapid growth. Subsequent HE staining and TUNEL staining showed obvious necrosis characteristics and higher proportion of positive apoptotic cell nucleus in high-dose irradiated tumors, but not nonirradiated tumors.
Conclusion
The APT signal intensity decreased after irradiation, which is earlier than the change of tumor volume. What is more, the decrease of APT signal intensity is more significant in high-dose group. Histological analysis showed obvious apoptosis and necrosis histological characteristic in irradiated tumor, which may explain the decrease of APT signal intensity. These results indicate that APT imaging has the potential to serve as a reliable biomarker for response assessment in NPC.






Similar content being viewed by others
References
Chen YP, Chan ATC, Le QT et al (2019) Nasopharyngeal carcinoma. Lancet. 394:64–80
Sun Y, Li WF, Chen NY et al (2016) Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 17:1509–1520
Tang LQ, Li CF, Li J et al (2016) Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst 108(1):djv291
Tang XR, Li YQ, Liang SB et al (2016) Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol 19:382–393
Zhu Q, Cai MY, Chen CL et al (2017) Tumor cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumor-infiltrating lymphocytes. Oncoimmunology 6:e1312240
Nishie A, Asayama Y, Ishigami K et al (2019) Amide proton transfer imaging to predict tumor response to neoadjuvant chemotherapy in locally advanced rectal cancer. J Gastroenterol Hepatol 34:140–146
Park KJ, Kim HS, Park JE et al (2016) Added value of amide proton transfer imaging to conventional and perfusion MR imaging for evaluating the treatment response of newly diagnosed glioblastoma. Eur Radiol 26:4390–4403
Park, JE, HS Kim, SY Park, et al. Identification of early response to anti-angiogenic therapy in recurrent glioblastoma: amide proton transfer-weighted and perfusion-weighted MRI compared with diffusion-weighted MRI. Radiology. 2020; 295:397-406.
Dula AN, Arlinghaus LR, Dortch RD et al (2013) Amide proton transfer imaging of the breast at 3 T: establishing reproducibility and possible feasibility assessing chemotherapy response. Magn Reson Med 70:216–224
Krikken E, Khlebnikov V, Zaiss M et al (2018) Amide chemical exchange saturation transfer at 7 T: a possible biomarker for detecting early response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res 20:51
Yu L, Li C, Luo X et al (2019) Differentiation of malignant and benign head and neck tumors with amide proton transfer-weighted MR imaging. Mol Imaging Biol 21:348–355
Bae YJ, Choi BS, Jeong WJ et al (2019) Amide proton transfer-weighted MRI in the diagnosis of major salivary gland tumors. Sci Rep 9:8349
Liu R, Jiang G, Gao P et al (2018) Non-invasive amide proton transfer imaging and ZOOM diffusion-weighted imaging in differentiating benign and malignant thyroid micronodules. Front Endocrinol (Lausanne) 9:747
Yuan, J, S Chen, AD King, et al. Amide proton transfer-weighted imaging of the head and neck at 3 T: a feasibility study on healthy human subjects and patients with head and neck cancer. NMR Biomed. 2014; 27:1239-1247.
Law BKH, King AD, Ai QY et al (2018) Head and Neck tumors: amide proton transfer MRI. Radiology 288:782–790
Qamar S, King AD, Ai QH et al (2020) Pre-treatment amide proton transfer imaging predicts treatment outcome in nasopharyngeal carcinoma. Eur Radiol 30(11):6339–6347
Qamar S, King AD, Ai QY et al (2019) Amide proton transfer MRI detects early changes in nasopharyngeal carcinoma: providing a potential imaging marker for treatment response. Eur Arch Otorhinolaryngol 276(2):505–512
Zhou J, Blakeley JO, Hua J et al (2008) Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med 60:842–849
Wen Z, Hu S, Huang F et al (2010) MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage 51:616–622
Zhou J, Zhu H, Lim M et al (2013) Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging 38:1119–1128
Wang S, Tryggestad E, Zhou T et al (2012) Assessment of MRI parameters as imaging biomarkers for radiation necrosis in the rat brain. Int J Radiat Oncol Biol Phys 83:e431–e436
Zhang J, Zhu W, Tain R et al (2018) Improved differentiation of low-grade and high-grade gliomas and detection of tumor proliferation using APT contrast fitted from z-spectrum. Mol Imaging Biol 20:623–631
Zhou J, Payen JF, Wilson DA et al (2003) Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 9:1085–1090
Lee DH, Heo HY, Zhang K et al (2017) Quantitative assessment of the effects of water proton concentration and water T1 changes on amide proton transfer (APT) and nuclear overhauser enhancement (NOE) MRI: the origin of the APT imaging signal in brain tumor. Magn Reson Med 77:855–863
Heo HY, Lee DH, Zhang Y et al (2017) Insight into the quantitative metrics of chemical exchange saturation transfer (CEST) imaging. Magn Reson Med 77:1853–1865
Zu Z (2018) Towards the complex dependence of MTRasym on T1w in amide proton transfer (APT) imaging. NMR Biomed 31:e3934
Zhou J, Heo HY, Knutsson L et al (2019) APT-weighted MRI: techniques, current neuro applications, and challenging issues. J Magn Reson Imaging 50:347–364
Zhou J, Hong X, Zhao X et al (2013) APT-weighted and NOE-weighted image contrasts in glioma with different RF saturation powers based on magnetization transfer ratio asymmetry analyses. Magn Reson Med 70:320–327
Zhang XY, Wang F, Li H et al (2017) Accuracy in the quantification of chemical exchange saturation transfer (CEST) and relayed nuclear overhauser enhancement (rNOE) saturation transfer effects. NMR Biomed 30(7):10.1002/nbm.3716. https://doi.org/10.1002/nbm.3716
Acknowledgements
The authors thank Professor Jinyuan Zhou (John Hopkins University) and Professor Yi Zhang (Zhejiang University) for technical assistance with APT imaging. We also thank engineer Weibo Chen (Philips Healthcare) for technical assistance with MRI. We thank engineer Xuefeng Zhang (Qilu hospital) and Jianzhen Wang (Qilu hospital) for animal irradiation.
Funding
This study was funded by the Special Fund for Taishan Scholar Project (Grant No. ts20190973) and the National Natural Science Foundation of China (Grant No. 81773228).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Qingxu Song, Pengxiang Chen, Cong Sun, and Xin Chen. The first draft of the manuscript was written by Qingxu Song, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, Q., Chen, P., Chen, X. et al. Dynamic Change of Amide Proton Transfer Imaging in Irradiated Nasopharyngeal Carcinoma and Related Histopathological Mechanism. Mol Imaging Biol 23, 846–853 (2021). https://doi.org/10.1007/s11307-021-01607-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01607-y