Abstract
Purpose
Magnetic particle imaging (MPI) is an emerging molecular imaging technique that directly detects iron nanoparticles distributed in living subjects. Compared with imaging iron with magnetic resonance imaging (MRI), MPI signal can be measured to determine iron content in specific regions. In this paper, the detection of iron-labeled macrophages associated with cancer by MRI and MPI was compared.
Procedures
Imaging was performed on 4T1 tumor-bearing mice 16–21 days post-cancer cell implantation, 24 h after intravenous injection of Ferucarbotran, a superparamagnetic iron oxide (SPIO) or Ferumoxytol, an ultra-small SPIO. Images of living mice were acquired on a 3T clinical MRI (General Electric, n = 6) or MPI (Magnetic Insight, n = 10) system. After imaging, tumors and lungs were removed, imaged by MPI and examined by histology.
Results
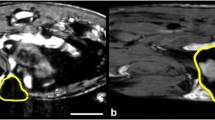
MRI signal voids were observed within all tumors. In vivo, MPI signals were observed in the tumors of 4 of 5 mice after the administration of each contrast agent and in all excised tumors. Signal voids visualized by MRI were more apparent in tumors of mice injected with Ferumoxytol than those that received Ferucarbotran; this was consistent with iron content measured by MPI. Signal voids relating to macrophage uptake of iron were not detected in lungs by MRI, since air also appears hypointense. In vivo, MPI could not differentiate between iron in the lungs vs the high signal from iron in the liver. However, once the lungs were excised, MPI signal was detectable and quantifiable. Histologic examination confirmed iron within macrophages present in the tumors.
Conclusions
MPI provides quantitative information on in vivo iron labeling of macrophages that is not attainable with MRI. The optimal iron nanoparticle for MPI in general is still under investigation; however, for MPI imaging of macrophages labeled in vivo by intravenous administration, Ferumoxytol nanoparticles were superior to Ferucarbotran.






Similar content being viewed by others
References
Arami H, Teeman E, Troksa A, Bradshaw H, Saatchi K, Tomitaka A, Gambhir SS, Häfeli UO, Liggitt D, Krishnan KM (2017) Tomographic magnetic particle imaging of cancer targeted nanoparticles. Nanoscale 9:18723–18730
Song G, Chen M, Zhang Y, Cui L, Qu H, Zheng X, Wintermark M, Liu Z, Rao J (2018) Janus iron oxides @ semiconducting polymer nanoparticle tracer for cell tracking by magnetic particle imaging. Nano Lett 18:182–189
Heyn C, Bowen CV, Rutt BK, Foster PJ (2005) Detection threshold of single SPIO-labeled cells with FIESTA. Magn Reson Med 53:312–320
Foster-Gareau P, Heyn C, Alejski A, Rutt BK (2003) Imaging single mammalian cells with a 1.5T clinical MRI scanner. Magn Reson Med 49:968–971
Makela AV, Gaudet JM, Foster PJ (2017) Quantifying tumor associated macrophages in breast cancer: a comparison of iron and fluorine-based MRI cell tracking. Sci Rep 7:42109
Gleich B, Weizenecker J (2005) Tomographic imaging using the nonlinear response of magnetic particles. Nature 435:1214
Oude Engberink RD, van der Pol SM A, Dopp EA et al (2007) Comparison of SPIO and USPIO for in vitro labeling of human monocytes: MR detection and cell function. Analysis 243:467–474
Obeid E, Nanda R, Fu YX, Olopade OI (2013) The role of tumor-associated macrophages in breast cancer progression. Int J Oncol 43:5–12
Kelly PM, Davison RS, Bliss E, McGee JO (1988) Macrophages in human breast disease: a quantitative immunohistochemical study. Br J Cancer 57:174–177
Doak GR, Schwertfeger KL, Wood DK (2018) Distant relations: macrophage functions in the metastatic niche. Trends Cancer 4:445–459
Daldrup-Link HE, Golovko D, Ruffell B, Denardo DG, Castaneda R, Ansari C, Rao J, Tikhomirov GA, Wendland MF, Corot C, Coussens LM (2011) MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin Cancer Res 17:5695–5704
Makela AV, Foster PJ (2018) Imaging macrophage distribution and density in mammary tumors and lung metastases using fluorine-19 MRI cell tracking. Magn Reson Med 80:1138–1147
Nejadnik H, Pandit P, Lenkov O, Lahiji AP, Yerneni K, Daldrup-Link HE (2019) Ferumoxytol can be used for quantitative magnetic particle imaging of transplanted stem cells. Mol Imaging Biol 21:465–472
Feraheme [Prescribing information] AMAG Pharmaceuticals Inc. Waltham, MA USA
Vivotrax [Datasheet] Magnetic Insight Inc. Alameda, CA USA
Dadashzadeh ER, Hobson M, Jr LHB et al (2013) Rapid spectrophotometric technique for quantifying Iron in cells labeled with superparamagnetic iron oxide nanoparticles: potential translation to the clinic. Contrast Media Mol Imaging 8:50–56
Zhang G-L, Zhang Y, Cao K-X, Wang X-M (2019) Orthotopic injection of breast cancer cells into the mice mammary fat pad. JoVE e58604
Abràmoff MD, Magalhães PJ, Ram SJ (2005) Image processing with ImageJ part II. Biophoton Int 11:36–43
Eberbeck D, Wiekhorst F, Wagner S, Trahms L (2011) How the size distribution of magnetic nanoparticles determines their magnetic particle imaging performance. Appl Phys Lett 98:182502
Corot C, Robert P, Idée JM, Port M (2006) Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev 58:1471–1504
Rosenblum LT, Kosaka N, Mitsunaga M, Choyke PL, Kobayashi H (2010) In vivo molecular imaging using nanomaterials: general in vivo characteristics of nano-sized reagents and applications for cancer diagnosis. Mol Membr Biol 27:274–285
Toth GB, Varallyay CG, Horvath A, Bashir MR, Choyke PL, Daldrup-Link HE, Dosa E, Finn JP, Gahramanov S, Harisinghani M, Macdougall I, Neuwelt A, Vasanawala SS, Ambady P, Barajas R, Cetas JS, Ciporen J, DeLoughery T, Doolittle ND, Fu R, Grinstead J, Guimaraes AR, Hamilton BE, Li X, McConnell H, Muldoon LL, Nesbit G, Netto JP, Petterson D, Rooney WD, Schwartz D, Szidonya L, Neuwelt EA (2017) Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int 92:47–66
Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res 46:6387–6392
Metz S, Bonaterra G, Rudelius M, Settles M, Rummeny EJ, Daldrup-Link HE (2004) Capacity of human monocytes to phagocytose approved iron oxide MR contrast agents in vitro. Eur Radiol 14:1851–1858
Makela AV, Foster PJ (2019) Preclinical 19F MRI cell tracking at 3 Tesla. Magn Reson Mater Phys Biol Med 32:123–132
De Jong WH, Hagens WI, Krystek P et al (2008) Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 29:1912–1919
Sabareeswaran A, Ansar EB, Harikrishna Varma PRV et al (2016) Effect of surface-modified superparamagnetic iron oxide nanoparticles (SPIONS) on mast cell infiltration: an acute in vivo study. Nanomed Nanotechnol Biol Med 12:1523–1533
Funding
This study was funded by the Canadian Institute of Health Research (PJF, 367445). AVM receives postdoctoral fellowship funding from the Natural Sciences and Engineering Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
JMG is an employee of Magnetic Insight Inc.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Makela, A.V., Gaudet, J.M., Schott, M.A. et al. Magnetic Particle Imaging of Macrophages Associated with Cancer: Filling the Voids Left by Iron-Based Magnetic Resonance Imaging. Mol Imaging Biol 22, 958–968 (2020). https://doi.org/10.1007/s11307-020-01473-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01473-0