Abstract
Introduction
Beef is the most consumed red meat in the United States, and the US is the largest producer and consumer of beef cattle globally. Feed is one of the largest input costs for the beef cattle industry, accounting for 40–60% of the total input costs. Identifying methods for improving feed efficiency in beef cattle herds could result in decreased cost to both producers and consumers, as well as increased animal protein available for global consumption.
Methods
In this study, rumen fluid was collected from low- (n = 14) and high-RFI (n = 15) steers. Rumen fluid was filtered through a 0.22 µM syringe filter, extracted using 0.1% formic acid in acetonitrile:water:methanol (2:2:1) and injected into the Dionex UltiMate 3000 UHPLC system with an Exactive Plus Orbitrap MS. Peaks were identified using MAVEN and analyzed using MetaboAnalyst 4.0 and SAS. Significance was determined using an α ≤ 0.05.
Results
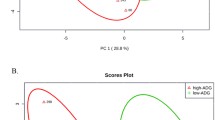
Eight metabolites were greater in low-RFI steers compared to high-RFI steers, including 3,4-dihydroxyphenylacetate, 4-pyridoxate, citraconate, hypoxanthine, succinate/methylmalonate, thymine, uracil, and xylose (P ≤ 0.05). These metabolites were predominantly involved in amino acid and lipid metabolism.
Conclusions
Rumen fluid metabolomes differ in steers of varying feed efficiencies. These metabolites may be used as biomarkers of feed efficiency, and may provide insight as to factors contributing to differences in feed efficiency that may be exploited to improve feed efficiency in beef cattle herds.




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Artegoitia, V. M., Foote, A. P., Lewis, R. M., & Freetly, H. C. (2017). Rumen fluid metabolomics analysis associated with feed efficiency on crossbred steers. Scientific Reports,7, 2864.
Arthur, P., Renand, G., & Krauss, D. (2001). Genetic and phenotypic relationships among different measures of growth and feed efficiency in young Charolais bulls. Livestock Production Science,68, 131–139.
Bechdel, S., Honeywell, H. E., Dutcher, R. A., & Knutsen, M. (1928). Synthesis of vitamin B in the rumen of the cow. Journal of Biological Chemistry,80, 231–238.
Benjamini, Y. and Hochberg, Y. (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological),57, 289–300.
Berg, J. M., Tymoczko, J. L., & Stryer, L. (2002). Biochemistry (5th ed.). New York: WH Freeman.
Blasi, P., Boyl, P. P., Ledda, M., Novelletto, A., Gibson, K. M., Jakobs, C., et al. (2002). Structure of human succinic semialdehyde dehydrogenase gene: Identification of promoter region and alternatively processed isoforms. Molecular Genetics and Metabolism,76, 348–362.
Chambers, M. C., Maclean, B., Burke, R., Amodei, D., Ruderman, D. L., Neumann, S., et al. (2012). A cross-platform toolkit for mass spectrometry and proteomics. Nature Biotechnology,30, 918.
Chambliss, K. L., & Gibson, K. M. (1992). Succinic semialdehyde dehydrogenase from mammalian brain: Subunit analysis using polyclonal antiserum. International Journal of Biochemistry,24, 1493–1499.
Chong, J., Soufan, O., Caraus, I., Xia, J., Li, C., Wishart, D. S., et al. (2018). Metaboanalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Research,46, W486–W494.
Clasquin, M.F., Melamud, E. and Rabinowitz, J.D. (2012) LC‐MS data processing with MAVEN: A metabolomic analysis and visualization engine. Current Protocols in Bioinformatics, 14.11. 1–14.11. 23.
Clemmons, B. A., Mihelic, R. I., Beckford, R. C., Powers, J. B., Melchior, E. A., McFarlane, Z. D., et al. (2017). Serum metabolites associated with feed efficiency in black angus steers. Metabolomics,13, 147.
Clemmons, B. A., Martino, C., Powers, J. B., Campagna, S. R., Voy, B. H., Donohoe, D. R., et al. (2019a). Rumen bacteria and serum metabolites predictive of feed efficiency phenotypes in beef cattle. Scientific Reports,9, 19265.
Clemmons, B. A., Martino, C., Schneider, L. G., Lefler, J., Embree, M. M., & Myer, P. R. (2019b). Temporal stability of the ruminal bacterial communities in beef steers. Scientific Reports,9, 9522.
ERS, U. (2019) Cattle and Beef Sector at a Glance in Knight, R. (Ed).
Fan, Z., Deng, J., Liu, G., Cai, H., He, J., Wu, M., et al. (2007). Effects of γ-aminobutyric acid on the performance and internal hormone levels in growing pigs. Chinese Journal of Animal Nutrition,19, 350–356.
Fan, H., Wu, Y., Zhou, X., Xia, J., Zhang, W., Song, Y., et al. (2015). Pathway-based genome-wide association studies for two meat production traits in Simmental cattle. Scientific Reports,5, 18389.
Ferrell, C., & Jenkins, T. (1984). Energy utilization by mature, nonpregnant, nonlactating cows of different types. Journal of Animal Science,58, 234–243.
Fontanesi, L. (2016). Metabolomics and livestock genomics: Insights into a phenotyping frontier and its applications in animal breeding. Animal Frontiers,6, 73–79.
Gnegy, M. E. (2012). Catecholamines. In S. T. Brady (Ed.), Basic neurochemistry (pp. 283–299). San Diego: Elsevier.
Goodrich, R., Garrett, J., Gast, D., Kirick, M., Larson, D., & Meiske, J. (1984). Influence of monensin on the performance of cattle. Journal of Animal Science,58, 1484–1498.
Henderson, G., Cox, F., Ganesh, S., Jonker, A., Young, W., Abecia, L., et al. (2015). Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Scientific Reports,5, 14567.
Kamphorst, J. J., Fan, J., Lu, W., White, E., & Rabinowitz, J. D. (2011). Liquid chromatography–high resolution mass spectrometry analysis of fatty acid metabolism. Analytical Chemistry,83, 9114–9122.
Kanehisa, M., Goto, S., Sato, Y., Kawashima, M., Furumichi, M., & Tanabe, M. (2013). Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Research,42, D199–D205.
Koch, R. M., Swiger, L. A., Chambers, D., & Gregory, K. E. (1963). Efficiency of feed use in beef cattle. Journal of Animal Science,22, 486–494.
Kong, R. S. G., Liang, G., Chen, Y., Stothard, P., & Guan, L. L. (2016). Transcriptome profiling of the rumen epithelium of beef cattle differing in residual feed intake. BMC Genomics,17, 592.
Krishnamachar, V., Subramanian, S., & Rao, M. R. (1964). Microbiological oxidation of the branched C 5-dicarboxylic acids. Archiv für Mikrobiologie,47, 338–343.
Leklem, J., & Machlin, L. (1991). Handbook of vitamins. New York: Marcel Decker Inc.
Leng, R., & Nolan, J. (1984). Nitrogen metabolism in the rumen. Journal of Dairy Science,67, 1072–1089.
Linkswiler, H., & Reynolds, M. S. (1950). Urinary and fecal elimination of B6 and 4-pyridoxic acid on three levels of intake. The Journal of Nutrition,41, 523–532.
Lu, W., Clasquin, M. F., Melamud, E., Amador-Noguez, D., Caudy, A. A., & Rabinowitz, J. D. (2010). Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Analytical Chemistry,82, 3212–3221.
McAllan, A., & Smith, R. (1973). Degradation of nucleic acid derivatives by rumen bacteria in vitro. British Journal of Nutrition,29, 467–474.
McAllan, A. B., Williams, A. P., Merry, R. J., & Smith, R. H. (1982). Effect of different levels of casein, with or without formaldehyde treatment, on carbohydrate metabolism between mouth and duodenum of steers. Journal of the Science of Food and Agriculture,33, 722–728.
McCormick, D. B. (2006). Vitamin B6. Present Knowledge in Nutrition,1, 269–277.
Merrill, A., & Burnham, F. (1990) Vitamin B-6. Present knowledge in nutrition (pp. 155–162). New York: Nutrition Foundation.
Montaño-Bermudez, M., Nielsen, M. K., & Deutscher, G. H. (1990). Energy requirements for maintenance of crossbred beef cattle with different genetic potential for milk. Journal of Animal Science,68, 2279–2288.
Myer, P. R., Smith, T. P. L., Wells, J. E., Kuehn, L. A., & Freetly, H. C. (2015). Rumen microbiome from steers differing in feed efficiency. PLoS ONE,10, e0129174.
Myer, P., Clemmons, B., Schneider, L., & Ault, T. (2019). Microbiomes in ruminant protein production and food security. CAB Reviews,14, 1–11.
Nafikov, R. A., & Beitz, D. C. (2007). Carbohydrate and lipid metabolism in farm animals. The Journal of Nutrition,137, 702–705.
Novais, F. J., Pires, P. R. L., Alexandre, P. A., Dromms, R. A., Iglesias, A. H., Ferraz, J. B. S., et al. (2019). Identification of a metabolomic signature associated with feed efficiency in beef cattle. BMC Genomics,20, 8.
Ogunade, I., & Schweickart, H. (2018). Effect of dietary monensin on rumen fluid metabolomic profile of beef cattle. Journal of Animal Science,96, 441–441.
Ogunade, I., Schweickart, H., Andries, K., Lay, J., & Adeyemi, J. (2018). Monensin alters the functional and metabolomic profile of rumen microbiota in beef cattle. Animals,8, 211.
Pearson, E., & Baldwin, B. (1981). D-xylose absorption in the adult bovine. The Cornell Veterinarian,71, 288–296.
Rabinowitz, J. D., & Kimball, E. (2007). Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Analytical Chemistry,79, 6167–6173.
Ryzlak, M. T., & Pietruszko, R. (1988). Human brain "high Km" aldehyde dehydrogenase: purification, characterization, and identification as NAD+ -dependent succinic semialdehyde dehydrogenase. Archives of Biochemistry and Biophysics,266, 386–396.
Thanh, V. T. K., & Ørskov, E. (2006). Causes of differences in urinary excretion of purine derivatives in buffaloes and cattle. Animal Science,82, 355–358.
Ushida, K., Miyazaki, A., & Kawashima, R. (1985). Effect of monensin on ruminal VFA and gas [methane] production of sheep fed high concentrate diet. Japanese Journal of Zootechnical Sciencem, 56, 822–826. https://doi.org/10.2508/chikusan.56.822.
Van Gylswyk, N. (1995). Succiniclasticum ruminis gen. nov., sp. nov., a ruminal bacterium converting succinate to propionate as the sole energy-yielding mechanism. International Journal of Systematic and Evolutionary Microbiology,45, 297–300.
Wang, D., Wang, C., Liu, H., Liu, J., & Ferguson, J. D. (2013). Effects of rumen-protected γ-aminobutyric acid on feed intake, lactation performance, and antioxidative status in early lactating dairy cows. Journal of Dairy Science,96, 3222–3227.
Weimer, P. J. (1998). Manipulating ruminal fermentation: A microbial ecological perspective. Journal of Animal Science,76, 3114–3122.
Weiss, W.P. and Ferreira, G. (2006). Water soluble vitamins for dairy cattle. Proceedings of the Tri-State Dairy Nutrition Conference Fort, Wayne, IN, pp. 51–63.
Westerhuis, J. A., Hoefsloot, H. C., Smit, S., Vis, D. J., Smilde, A. K., van Velzen, E. J., et al. (2008). Assessment of PLSDA cross validation. Metabolomics,4, 81–89.
Wirth, R., Kádár, G., Kakuk, B., Maróti, G., Bagi, Z., Szilágyi, Á., et al. (2018). The planktonic core microbiome and core functions in the cattle rumen by next generation sequencing. Frontiers in Microbiology,9, 2285.
Yost, W. M., Young, J. W., Schmidt, S. P., & McGilliard, A. D. (1977). Gluconeogenesis in ruminants: Propionic acid production from a high-grain diet fed to cattle. The Journal of Nutrition,107, 2036–2043.
Acknowledgements
This study was supported by Ascus Biosciences, Inc. (Grant No. A17-0146–003) and USDA-NIFA Hatch/Multistate Project W4177—TEN00538—Enhancing the Competitiveness and Value of U.S. Beef; Accession Number: 1016984. The authors thank the staff at the Plateau Research and Education Center in Crossville, TN for their technical assistance.
Author information
Authors and Affiliations
Contributions
BAC performed research, analyzed data, and wrote the paper. JBP contributed analytical tools and analyzed data. SRC contributed analytical tools and analyzed data. TBS analyzed data and wrote segments of the paper. MME conceived of or designed study, performed research, and analyzed data. PRM conceived of or designed study, performed research, analyzed data, and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
BA Clemmons, JB Powers, SR Campagna, TB Seay, and PR Myer declare that they have no conflict of interest. MM Embree is the Co-Founder and Chief Science Officer at Ascus Biosciences, Inc.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Clemmons, B.A., Powers, J.B., Campagna, S.R. et al. Rumen fluid metabolomics of beef steers differing in feed efficiency. Metabolomics 16, 23 (2020). https://doi.org/10.1007/s11306-020-1643-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-020-1643-x