Abstract
Introduction
Secreted molecules could be correlated with the potential of embryonic development. The development of new technologies, such as mass spectrometry (MS), has enabled analyzes in culture medium to favor the determination of embryos viability in order to improve embryo selection.
Objectives
To perform a non-invasive characterization of the secretome of in vitro produced embryos with different kinetics of cleavage and in different stages of development to obtain specific patterns based on embryonic phenotype through MALDI–TOF–MS.
Methods
Bovine embryos were produced in vitro by standard protocols. The zygotes were transferred to individual culture medium and divided into two groups: Fast [4 cells-22 hours past the beginning of culture (hpc)] and Slow (2 cells-22 hpc). Culture media drops were collected at 22, 96 and 168 hpc. Analysis of embryonic secretome was made by MALDI–TOF–MS after extractions of the metabolites. Spectra were acquired in positive ionization mode. Univariate (Fold-change) and multivariate (Partial Least Squares Discriminants Analysis) analyses were performed by the online software Metaboanalyst.
Results
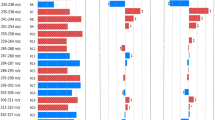
It was demonstrated that embryos with different kinetics have different spectrometric profiles during embryonic development. Moreover, secreted molecules in each developmental stage are differentially represented in embryos with different kinetics, and are related to specific pathways such as lipid and amino acids metabolism and cell proliferation.
Conclusion
We propose that the analysis of culture media by MALDI–TOF–MS can be used for qualitative characterization of bovine embryos, allowing the identification of key molecules during in vitro culture.

Similar content being viewed by others
References
Alhonen-Hongisto, L., Hirvonen, A., Sinervirta, R., & Jänne, J. (1987). Cadaverine supplementation during a chronic exposure to difluoromethylornithine allows an overexpression, but prevents gene amplification, of ornithine decarboxylase in L1210 mouse leukaemia cells. Biochemical Journal, 247(3), 651–655.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911–917.
Bohrer, R. C., Che, L., Gonçalves, P. B. D., Duggavathi, R., & Bordignon, V. (2013). Phosphorylated histone H2A.x in porcine embryos produced by IVF and somatic cell nuclear transfer. Reproduction,. doi:10.1530/REP-13-0271.
Booth, P. J., Watson, T. J., & Leese, H. J. (2007). Prediction of porcine blastocyst formation using morphological, kinetic, and amino acid depletion and appearance criteria determined during the early cleavage of in vitro-produced embryos. Biology of Reproduction, 5, 765–779.
Brison, D. R., Houghton, F. D., Falconer, D., Roberts, S. A., Hawkhead, J., Humpherson, P. G., & Leese, H. J. (2004). Identification of viable embryos in IVF by non-invasive measurement of amino acid trunover. Human Reproduction, 19, 2319–2324.
Camargo, M., Intasqui, P., Lima, C. B., Montani, D. A., Nichi, M., Pilau, E. J., et al. (2014). MALDI–TOF fingerprinting of seminal plasma lipids in the study of human male infertility. Lipids,. doi:10.1007/s11745-014-3922-7.
Cánepa, M. J., Ortega, N. M., Monteleone, M. C., Mucci, N., Kaiser, G. G., Brocco, M., & Mutto, A. (2014). Expression profile of genes as indicators of developmental competence and quality of in vitro fertilization and somatic cell nuclear transfer bovine embryos. PLoS One,. doi:10.1371/journal.pone.0108139.
Carrocera, S., Caamaño, J. N., Trigal, B., Martín, D., & Díez, C. (2015). Developmental kinetics of in vitro-produced bovine embryos: An aid for making decisions. Theriogenology,. doi:10.1016/j.theriogenology.2015.10.028.
Cortezzi, S. S., Garcia, J. S., Ferreira, C. R., Braga, D. P. A. F., Figueira, R. C. S., Iaconelli, A, Jr, et al. (2011). Secretome of the preimplantation human embryo by bottom-up label-free proteomics. Analytical and Bioanalytical Chemistry, 401, 1331–1339.
Ferreira, C. R., Pirro, V., Eberlin, L. S., Hallett, J. E., & Cooks, R. G. (2012). Developmental phases of individual mouse preimplantation embryos characterized by lipid signatures using desorption electrospray ionization mass spectrometry. Analytical and Bioanalytical Chemistry, 404(10), 2915–2926.
Garcia, S. M., Marinho, L. S. R., Lunardelli, P. A., Seneda, M. M., & Meirelles, F. V. (2015). Developmental block and programmed cell death in bosindicus embryos: Effects of protein supplementation source and developmental kinetics. PLoS One,. doi:10.1371/journal.pone.0119463.
Garcia-Herreros, M., Aparicio, I. M., Rath, D., Fair, T., & Lonergan, P. (2012). Differential glycolytic and glycogenogenic transduction pathways in male and female bovine embryos produced in vitro. Reproduction, Fertility and Development, 24(2), 344–352.
Gardner, D. K., & Harvey, A. J. (2015). Blastocyst metabolism. Reproduction, Fertility, and Development, 27, 638–654.
Gardner, D. K., Wale, P. L., Collins, R., & Lane, M. (2011). Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Human Reproduction, 26(8), 1981–1986.
Goldstein, J. L., & Brown, M. S. (1990). Regulation of the mevalonate pathway. Nature, 343(6257), 425–430.
Grochowski, L. L., Xu, H., & White, R. H. (2006). Methanocaldococcusjannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate. Journal of Bacteriology, 188(9), 3192–3198.
Hamana, K., Matsuzaki, S., Hosaka, K., & Yamashita, S. (1989). Interconversion of polyamines in wild-type strains and mutants of yeasts and the effects of polyamines on their growth. FEMS Microbiology Letters, 52, 231–236.
Hansen, J. M., & Harris, C. (2015). Glutathione during embryonic development. Biochimica et Biophysica Acta (BBA): General Subjects, 1850(8), 1527–1542.
Hinson, D. D., Chambliss, K. L., Toth, M. J., Tanaka, R. D., & Gibson, K. M. (1997). Post-translational regulation of mevalonate kinase by intermediates of the cholesterol and nonsterol isoprene biosynthetic pathways. Journal of Lipid Research, 38(11), 2216–2223.
Holm, P., Booth, P. J., & Callesen, D. H. (2002). Kinetics of early in vitro development of bovine in vivo- and in vitro-derived zygotes produced and/or cultured in chemically defined or serum-containing media. Reproduction, 123, 553–565.
Hölttä, E., & Pohjanpelto, P. (1982). Polyamine dependence of Chinese hamster ovary cells in serum-free culture is due to deficient arginase activity. Biochimica et Biophysica Acta, 721, 321–327.
Huwiler, A., Kolter, T., Pfeilschifter, J., & Sandhoff, K. (2000). Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochimica et Biophysica Acta, 1485, 63–99.
Katayama, M., Rieke, A., Cantley, T., Murphy, C., Dowell, L., Sutovsky, P., & Day, B. N. (2007). Improved fertilization and embryo development resulting in birth of live piglets after intracytoplasmic sperm injection and in vitro culture in a cysteine-supplemented medium. Theriogenology, 67(4), 835–847.
Katz-Jaffe, M. G., & Mcreynolds, S. (2013). Embryology in the era of proteomics. Fertility and Sterility, 99(4), 1073–1077.
Katz-Jaffe, M. G., McReynolds, S., Gardner, D. K., & Schoolcraft, W. B. (2009). The role of proteomics in defining the human embryonic secretome. Molecular Human Reproduction, 15, 271–277.
Katz-Jaffe, M. G., Schoolcraft, W. B., & Gardner, D. K. (2006). Analysis of protein expression (secretome) by human and mouse preimplantation embryos. Fertility and Sterility,. doi:10.1016/j.fertnstert.2006.05.022.
Kim, S. H., Zhao, M. H., Liang, S., Cui, X. S., & Kim, N. H. (2015). Inhibition of cathepsin B activity reduces apoptosis by preventing cytochrome c release from mitochondria in porcine parthenotes. Journal of Reproduction and Development, 61(4), 261–268.
Kunert, J. (1985). Metabolism of sulfur-containing amino acids in the dermatophyte Microsporum gypseum. II. Acidic amino acid derivatives. Journal of Basic Microbiology, 25(2), 111–118.
Leão, B. C., Rocha-Frigoni, N. A., Cabral, E. C., Franco, M. F., Ferreira, C. R., Eberlin, M. N., et al. (2014). Membrane lipid profile monitored by mass spectrometry detected differences between fresh and vitrified in vitro-produced bovine embryos. Zygote,. doi:10.1017/S0967199414000380.
Lee, Y. S. L., Thouas, G. A., & Gardner, D. K. (2015). Developmental kinetics of cleavage stage mouse embryos are related to their subsequent carbohydrate and amino acid utilization at the blastocyst stage. Human Reproduction, 30(3), 543–552.
Leese, H. J. (2002). Quiet please, do not disturb: A hypothesis of embryo metabolism and viability. BioEssays, 24, 845–849.
Li, X. X., Lee, K. B., Lee, J. H., Kim, K. J., Kim, E. Y., Han, K. W., et al. (2014). Glutathione and cysteine enhance porcine preimplantation embryo development in vitro after intracytoplasmic sperm injection. Theriogenology, 81(2), 309–314.
Market Velker, B. A., Denomme, M. M., & Mann, M. R. (2012). Loss of genomic imprinting in mouse embryos with fast rates of preimplantation development in culture. Biology of Reproduction, 86(143), 1–16.
Merrill, A. H., Schmelz, E. M., Dillehay, D. L., Spiegel, S., Shayman, J. A., Schroeder, J. J., et al. (1997). Sphingolipids—the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicology and Applied Pharmacology, 142, 208–225.
Milazzotto, M. P., Goissis, M. D., Chitwood, J. L., Annes, K., Soares, C. A., Ispada, J., et al. (2016). Early cleavages influence the molecular and the metabolic pattern of individually cultured bovine blastocysts. Mol: Molecular Reproduction and Development. doi:10.1002/mrd.22619.
Mizugishi, K., Li, C., Olivera, A., Bielawski, J., Bielawska, A., Deng, C.-X., & Proia, R. L. (2007). Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. The Journal of Clinical Investigation, 117(10), 2993–3006.
Muñoz, M., Uyar, A., Correia, E., Díez, C., Fernandez-Gonzalez, A., Caamaño, J. N., et al. (2014). Prediction of pregnancy viability in bovine in vitro-produced embryos and recipient plasma with Fourier transform infrared spectroscopy. Journal of Dairy Science, 97(9), 5497–5507.
Nes, W. D. (2011). Biosynthesis of cholesterol and other sterols. Chemical Reviews,. doi:10.1021/cr200021m.
Parrish, J. J., Susko-Parrish, J. L., Winer, M. A., & First, N. L. (1988). Capacitation of bovine sperm by heparin. Biology of Reproduction, 38, 1171–1180.
Schiller, J., Arnhold, J., Benard, S., Muller, M., Reichl, S., & Arnold, K. (1999). Lipid analysis by matrix-assisted laser desorption and ionization mass spectrometry: a methodological approach. Analytical Biochemistry, 267(1), 46–56.
Sevier, C. S., & Kaiser, C. A. (2002). Formation and transfer of disulphide bonds in living cells. Nature Reviews Molecular Cell Biology, 3(11), 836–847.
Sturmey, R. G., Bermejo-Alvarez, P., Gutierrez-Adan, A., Rizos, D., Leese, H. J., & Lonergan, P. (2010). Amino acid metabolism of bovine blastocysts: a biomarker of sex and viability. Molecular Reproduction and Development, 77(3), 285–296.
Sudano, M. J., Santos, V. G., Tata, A., Ferreira, C. R., Paschoal, D. M., Machado, R., et al. (2012). Phosphatidylcholine and sphingomyelin profiles vary in bostaurusindicus and bostaurustaurus in vitro- and in vivo-produced blastocysts. Biology of Reproduction,. doi:10.1095/biolreprod.112.10289.
Sugimura, S., Akai, T., Hashiyada, Y., Somfai, T., Inaba, Y., Hirayama, M., et al. (2012). Promising system for selecting healthy in vitro-fertilized embryos in cattle. PLoS One,. doi:10.1371/journal.pone.0036627.
Sutton-Mcdowall, M. L., Feil, D., Robker, R. L., Thompson, J. G., & Dunning, K. R. (2012). Utilization of endogenous fatty acid stores for energy production in bovine preimplantation embryos. Theriogenology, 77(8), 1632–1641.
Tasseva, G., Bai, H. D., Davidescu, M., Haromy, A., Michelakis, E., & Vance, J. E. (2013). Phosphatidylethanolamine deficiency in Mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. Journal of Biological Chemistry, 288(6), 4158–4173.
Urbanski, J. P., Johnson, M. T., Craig, D. D., Potter, D. L., Gardner, D. K., & Thorsen, T. (2008). Noninvasive metabolic profiling using microfluidics for analysis of single preimplantation embryos. Analytical Chemistry, 80(17), 6500–6507.
Vajta, G., Peura, T. T., Holm, P., Páldi, A., Greve, T., Trounson, A. O., & Callesen, H. (2000). New method for culture of zona-included or zona-free embryos: the well of the well (WOW) system. Molecular Reproduction and Development, 55, 256–264.
Vandaele, L., Mateusen, B., Maes, D. G., de Kruif, A., & Van Soom, A. (2007). Temporal detection of caspase-3 and-7 in bovine in vitro produced embryos of different developmental capacity. Reproduction, 133(4), 709–718.
Xia, J., Mandal, R., Sinelnikov, I., Broadhurst, D., & Wishart, D. S. (2012). MetaboAnalyst 2.0: A comprehensive server for metabolomic data analysis. Nucleic Acids Research,. doi:10.1093/nar/gks374.
Xia, J., Psychogios, N., Young, N., & Wishart, D. S. (2009). MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Research,. doi:10.1093/nar/gkp356.
Acknowledgments
The authors would like to thank São Paulo Research Foundation (FAPESP) (grant number 2012/10351-2) and Coordination for the Improvement of Higher Education Personnel (CAPES) for financial support. The authors would also like to thank the Multiuser Mass Spectrometry Laboratory at Sao Paulo Federal University, Fernanda Bertuccez Cordeiro and Augusto Azzolini for the experimental support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest to declare.
Ethical approval
All procedures and protocols were performed in accordance with the Ethical Principles in Animal Research set forth by the Brazilian College of Animal Experimentation, with approval from “Ethic Committee in the Use of Animal” of Universidade Federal do ABC (Protocol No. 008/2014).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
dos Santos, É.C., de Lima, C.B., Annes, K. et al. Noninvasive characterization of metabolites secreted in culture media by bovine embryos during in vitro production. Metabolomics 12, 94 (2016). https://doi.org/10.1007/s11306-016-1029-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-016-1029-2