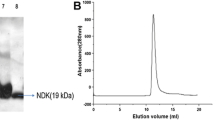
Abstract
Leishmania braziliensis is a pathogenic protozoan parasite that causes American Tegumentary Leishmaniasis (ATL), an important tropical neglected disease. ENTPDases are nucleotidases that hydrolyze intracellular and/or extracellular nucleotides. ENTPDases are known as regulators of purinergic signalling induced by extracellular nucleotides. Leishmania species have two isoforms of ENTPDase, and, particularly, ENTPDase2 seems to be involved in infectivity and virulence. In this study, we conducted the heterologous expression and biochemical characterization of the recombinant ENTPDase2 of L. braziliensis (rLbNTPDase2). Our results show that this enzyme is a canonical ENTPDase with apyrase activity, capable of hydrolysing triphosphate and diphosphate nucleotides, and it is dependent on divalent cations (calcium or magnesium). Substrate specificity was characterized as UDP>GDP>ADP>GTP>ATP=UTP. The enzyme showed optimal activity at a neutral to basic pH and was partially inhibited by suramin and DIDS. Furthermore, the low apparent Km for ADP suggests that the enzyme may play a role in adenosine-mediated signalling. The biochemical characterization of this enzyme can open new avenues for using LbNTPDase2 as a drug target.
Graphical abstract






Similar content being viewed by others
Data Availability
Not applicable
References
Reithinger R, Dujardin JC, Louzir H et al (2007) Cutaneous leishmaniasis. Lancet Infect Dis 7:581–596. https://doi.org/10.1016/S1473-3099(07)70209-8
Volpedo G, Pacheco-Fernandez T, Holcomb EA et al (2021) Mechanisms of immunopathogenesis in cutaneous leishmaniasis and post kala-azar dermal leishmaniasis (PKDL). Front Cell Infect Microbiol 11:1–16. https://doi.org/10.3389/fcimb.2021.685296
Knowles AF (2011) The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signal 7:21–45. https://doi.org/10.1007/s11302-010-9214-7
Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362:299–309. https://doi.org/10.1007/s002100000309
Robson SC, Sévigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2:409–430. https://doi.org/10.1007/s11302-006-9003-5
Zimmermann H, Zebisch M, Sträter N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8:437–502. https://doi.org/10.1007/s11302-012-9309-4
Cao W, Fang F, Gould T et al (2020) Ecto-NTPDase CD39 is a negative checkpoint that inhibits follicular helper cell generation. J Clin Invest 130:3422–3436. https://doi.org/10.1172/JCI132417
Corriden R, Chen Y, Inoue Y et al (2008) Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem 283:28480–28486. https://doi.org/10.1074/jbc.M800039200
Jhandier MN, Kruglov EA, Lavoie ÉG et al (2005) Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem 280:22986–22992. https://doi.org/10.1074/jbc.M412371200
Langston HP, Ke Y, Gewirtz AT et al (2003) Secretion of IL-2 and IFN- γ , But Not IL-4, by antigen-specific T cells requires extracellular ATP. J Immunol 170:2962–2970. https://doi.org/10.4049/jimmunol.170.6.2962
Marcus AJ, Broekman MJ, Drosopoulos JHF et al (1997) The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest 99:1351–1360. https://doi.org/10.1172/JCI119294
Leite PM, Gomes RS, Figueiredo AB et al (2012) Ecto-nucleotidase activities of promastigotes from Leishmania ( Viannia ) braziliensis relates to parasite infectivity and disease clinical outcome. PLoS Negl Trop Dis 6:e1850. https://doi.org/10.1371/journal.pntd.0001850
Asai T, O’Sullivan JW, Tatibana M (1983) A potent nucleoside triphosphate hydrolase from the parasitic protozoan Toxoplasma gondii. J Biol Chem 258:6816–6822
Asai T, Miura S, Sibley LD et al (1995) Biochemical and molecular characterization of nucleoside triphosphate hydrolase isozymes from the parasitic protozoan Toxoplasma gondii. J Biol Chem 270:11391–11397. https://doi.org/10.1074/jbc.270.19.11391
Vasconcelos EG, Ferreira ST, De Carvalho TMU et al (1996) Partial purification and immunohistochemical localization of ATP diphosphohydrolase from Schistosoma mansoni: Immunological cross-reactivities with potato apyrase and Toxoplasma gondii nucleoside triphosphate hydrolase. J Biol Chem 271:22139–22145. https://doi.org/10.1074/jbc.271.36.22139
Berrêdo-Pinho M, Peres-Sampaio CE, Chrispim PPM et al (2001) A mg-dependent ecto-ATPase in Leishmania amazonensis and its possible role in adenosine acquisition and virulence. Arch Biochem Biophys 391:16–24. https://doi.org/10.1006/abbi.2001.2384
Pinheiro CM, Martins-Duarte ES, Ferraro RB et al (2006) Leishmania amazonensis: biological and biochemical characterization of ecto-nucleoside triphosphate diphosphohydrolase activities. Exp Parasitol 114:16–25. https://doi.org/10.1016/j.exppara.2006.02.007
Paes-Vieira L, Rocco-Machado N, Freitas-Mesquita AL et al (2021) Differential regulation of E-NTPdases during Leishmania amazonensis lifecycle and effect of their overexpression on parasite infectivity and virulence. Parasitol Int 85:102423. https://doi.org/10.1016/j.parint.2021.102423
Fietto JLR, DeMarco R, Nascimento IP et al (2004) Characterization and immunolocalization of an NTP diphosphohydrolase of Trypanosoma cruzi. Biochem Biophys Res Commun 316:454–460. https://doi.org/10.1016/j.bbrc.2004.02.071
Mariotini-Moura C, Bastos MS, de Castro FF et al (2014) Trypanosoma cruzi nucleoside triphosphate diphosphohydrolase 1 (TcNTPDase-1) biochemical characterization, immunolocalization and possible role in host cell adhesion. Acta Trop 130:140–147. https://doi.org/10.1016/j.actatropica.2013.11.008
Rezende-Soares FA, Carvalho-Campos C, Marques MJ et al (2010) Cytochemical localization of ATP diphosphohydrolase from Leishmania (Viannia) braziliensis promastigotes and identification of an antigenic and catalytically active isoform. Parasitology 137:773–783. https://doi.org/10.1017/S0031182009991661
Porcino GN, Carvalho-Campos C, Maia ACRG et al (2012) Leishmania (Viannia) braziliensis nucleoside triphosphate diphosphohydrolase (NTPDase 1): localization and in vitro inhibition of promastigotes growth by polyclonal antibodies. Exp Parasitol 132:293–299. https://doi.org/10.1016/j.exppara.2012.08.009
Vasconcellos RDS, Mariotini-Moura C, Gomes RS et al (2014) Leishmania infantum ecto-nucleoside triphosphate diphosphohydrolase-2 is an apyrase involved in macrophage infection and expressed in infected dogs. PLoS Negl Trop Dis 8:e3309. https://doi.org/10.1371/journal.pntd.0003309
Sansom FM, Ralton JE, Sernee MF et al (2014) Golgi-located NTPDase1 of Leishmania major is required for lipophosphoglycan elongation and normal lesion development whereas secreted NTPDase2 is dispensable for virulence. PLoS Negl Trop Dis 8:e3402. https://doi.org/10.1371/journal.pntd.0003402
Borges-Pereira L, Meissner KA, Wrenger C, Garcia CRS (2017) Plasmodium falciparum GFP-E-NTPDase expression at the intraerythrocytic stages and its inhibition blocks the development of the human malaria parasite. Purinergic Signal 13:267–277. https://doi.org/10.1007/s11302-017-9557-4
Bisaggio DFR, Peres-Sampaio CE, Meyer-Fernandes JR, Souto-Padrón T (2003) Ecto-ATPase activity on the surface of Trypanosoma cruzi and its possible role in the parasite-host cell interaction. Parasitol Res 91:273–282. https://doi.org/10.1007/s00436-003-0965-8
Maioli T, Takane EMR, Arantes E et al (2004) Immune response induced by New World Leishmania species in C57BL / 6 mice. Parasitol Res 94:207–212. https://doi.org/10.1007/s00436-004-1193-6
Marques-da-silva EDA, Camargos J, Oliveira D, Afonso C (2008) Extracellular nucleotide metabolism in Leishmania: influence of adenosine in the establishment of infection. Microbes Infect 10:850–857. https://doi.org/10.1016/j.micinf.2008.04.016
Gomes RS, de Carvalho LCF, de Souza VR et al (2015) E-NTPDase (ecto-nucleoside triphosphate diphosphohydrolase) of Leishmania amazonensis inhibits macrophage activation. Microbes Infect 17:295–303. https://doi.org/10.1016/j.micinf.2014.12.009
Detoni ML, Fessel MR, Maia ACRG et al (2013) An antigenic domain of the Leishmania amazonensis nucleoside triphosphate diphosphohydrolase (NTPDase 1) is associated with disease progression in susceptible infected mice. Parasitol Res 112:2773–2782. https://doi.org/10.1007/s00436-013-3445-9
Maia ACRG, Porcino GN, Detoni ML et al (2019) Leishmania infantum amastigote nucleoside triphosphate diphosphohydrolase 1 (NTPDase 1): its inhibition as a new insight into mode of action of pentamidine. Exp Parasitol 200:1–6. https://doi.org/10.1016/j.exppara.2019.03.003
da Silva W, da Rocha TN, de Melo AJ et al (2020) ENTPDases from pathogenic trypanosomatids and purinergic signaling: shedding light towards biotechnological applications. Curr Top Med Chem 21:213–226. https://doi.org/10.2174/1568026620666201005125146
de Souza ACA, de Castro RB, dos Santos YL et al (2020) High performance of ELISA test using recombinant rLiNTPDase2 from Leishmania infantum: a phase II diagnosis of canine visceral leishmaniasis. Acta Trop 209:105535. https://doi.org/10.1016/j.actatropica.2020.105535
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windowns 95/98/NT. Nucleic Acids Symp Ser 41:95–98. https://doi.org/10.14601/phytopathol_mediterr-14998u1.29
South J, Blass B (2001) The future of modern genomics. Blackwell, London.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/j.cj.2017.04.003
Ekman P, Jager O (1993) Quantification of subnanomolar amounts of phosphate bound to seryl and threonyl residues in phosphoproteins using alkaline hydrolysis and malachite green. Anal Biochem 214:138–141. https://doi.org/10.1006/abio.1993.1468
Barros FS, De Menezes LF, Pinheiro AAS et al (2000) Ectonucleotide diphosphohydrolase activities in Entamoeba histolytica. Arch Biochem Biophys 375:304–314. https://doi.org/10.1006/abbi.1999.1592
Meyer-Fernandes JR, Dutra PML, Rodrigues CO et al (1997) Mg-dependent ecto-ATPase activity in Leishmania tropica. Arch Biochem Biophys 341:40–46. https://doi.org/10.1006/abbi.1997.9933
Freitas-Mesquita AL, Meyer-Fernandes JR (2017) 3′nucleotidase/nuclease in protozoan parasites: molecular and biochemical properties and physiological roles. Exp Parasitol 179:1–6. https://doi.org/10.1016/j.exppara.2017.06.001
Gottlieb M, Dwyer DM (1983) Evidence for Distinct 5’- and 3’-nucleotidase activities in the surface membrane fraction of Leishmania donovani promastigotes. Mol Biochem Parasitol 7:303–317. https://doi.org/10.1016/0166-6851(83)90013-0
Quiñonez-Díaz L, Mancilla-Ramírez J, Avila-García M et al (2012) Effect of ambient temperature on the clinical manifestations of experimental diffuse cutaneous leishmaniasis in a rodent model. Vector-Borne Zoonotic Dis 12:851–860. https://doi.org/10.1089/vbz.2011.0844
Chen BC, Wan-Wan L (1997) Inhibition of ecto-ATPase by the P2 purinoceptor agonists, ATPγS, α,β-methylene-ATP, and AMP-PNP, in endothelial cells. Biochem Biophys Res Commun 233:442–446. https://doi.org/10.1006/bbrc.1997.6478
Santos RF, Bastos MS, Guedes PMM et al (2009) Influence of ecto-nucleoside triphosphate diphosphohydrolase activity on Trypanosoma cruzi infectivity and virulence. PLoS Negl Trop Dis 3:e387. https://doi.org/10.1371/journal.pntd.0000387
Kukulski F, Levesque SA, Lavoie EG et al (2005) Comparative hrydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal 1:193–204. https://doi.org/10.1007/s11302-005-0383-8
Frick L, Hinterland L, Renner K et al (2022) Acidic microenvironments found in cutaneous Leishmania lesions curtail NO-dependent antiparasitic macrophage activity. Front Immunol 13:1–10. https://doi.org/10.3389/fimmu.2022.789366
Ivanenkov VV, Murphy-Piedmonte DM, Kirley TL (2003) Bacterial expression, characterization, and disulfide bond determination of soluble human NTPDase6 (CD39L2) nucleotidase: implications for structure and function. Biochemistry 42:11726–11735. https://doi.org/10.1021/bi035137r
Murphy-Piedmonte DM, Crawford PA, Kirley TL (2005) Bacterial expression, folding, purification and characterization of soluble NTPDase5 (CD39L4) ecto-nucleotidase. Biochim Biophys Acta - Proteins Proteomics 1747:251–259. https://doi.org/10.1016/j.bbapap.2004.11.017
Moeckel D, Jeong SS, Sun X et al (2014) Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. Sci Transl Med 6:248ra105. https://doi.org/10.1126/scitranslmed.3009246
Grinthal A, Guidotti G (2000) Substitution of His59 converts CD39 apyrase into an ADPase in a quaternary structure dependent manner. Biochemistry 39:9–16. https://doi.org/10.1021/bi991751k
Hicks-Berger CA, Chadwick BP, Frischauf AM, Kirley TL (2000) Expression and characterization of soluble and membrane-bound human nucleoside triphosphate diphosphohydrolase 6 (CD39L2). J Biol Chem 275:34041–34045. https://doi.org/10.1074/jbc.M004723200
Krug U, Zebisch M, Krauss M, Sträter N (2012) Structural insight into activation mechanism of Toxoplasma gondii nucleoside triphosphate diphosphohydrolases by disulfide reduction. J Biol Chem 287:3051–3066. https://doi.org/10.1074/jbc.M111.294348
Zebisch M, Sträter N (2008) Structural insight into signal conversion and inactivation by NTPDase2 in purinergic signaling. Proc Natl Acad Sci U S A 105:6882–6887. https://doi.org/10.1073/pnas.0802535105
Burnstock G, Boeynaems JM (2014) Purinergic signalling and immune cells. Purinergic Signal 10:529–564. https://doi.org/10.1007/s11302-014-9427-2
Bours MJL, Swennen ELR, Di Virgilio F et al (2006) Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 112:358–404. https://doi.org/10.1016/j.pharmthera.2005.04.013
Dosch M, Gerber J, Jebbawi F, Beldi G (2018) Mechanisms of ATP release by inflammatory cells. Int J Mol Sci 19:1–16. https://doi.org/10.3390/ijms19041222
Pellegatti P, Raffaghello L, Bianchi G et al (2008) Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One 3:1–9. https://doi.org/10.1371/journal.pone.0002599
Di Virgilio F, Vuerich M (2015) Purinergic signaling in the immune system. Auton Neurosci Basic Clin 191:117–123. https://doi.org/10.1016/j.autneu.2015.04.011
Panupinthu N, Zhao L, Possmayer F et al (2007) P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. J Biol Chem 282:3403–3412. https://doi.org/10.1074/jbc.M605620200
Haskó G, Pacher P (2012) Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol 32:865–869. https://doi.org/10.1161/ATVBAHA.111.226852
da Silva W, Ribeiro IC, de Agripino JM et al (2023) Leishmania infantum NTPDase1 and NTPDase2 play an important role in infection and nitric oxide production in macrophages. Acta Trop 237:106732. https://doi.org/10.1016/j.actatropica.2022.106732
Marr JJ, Berens RL, Nelson DJ (1978) Purine metabolism in Leishmania donovani and Leishmania braziliensis. Biochim Biophys Acta 544:360–371. https://doi.org/10.1016/0304-4165(78)90104-6
Acknowledgements
The authors gratefully acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the fellowship granted to JLRF and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for student’s scholarships.
Funding
This study was sponsored in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—grant code 001.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Material preparation, data collection, and analysis were performed by NdRTP. JVBdM, ICR, RBdC, WdS, ACAdS, and VHFdS. The first draft of the manuscript was written by NdRTP; the first revision was done by JLRF, and all authors commented on previous versions of the manuscript. The data and analyses were carefully reviewed by JLRF. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflicts of interest
Nancy da Rocha Torres Pavione declares that she has no conflict of interest.
João Victor Badaró de Moraes declares that he has no conflict of interest.
Isadora Cunha Ribeiro declares that she has no conflict of interest.
Raissa Barbosa de Castro declares that she has no conflict of interest.
Walmir da Silva declares that he has no conflict of interest.
Anna Cláudia Alves de Souza declares that she has no conflict of interest.
Victor Hugo Ferraz da Silva declares that he has no conflict of interest.
Raphael de Souza Vasconcellos declares that he has no conflict of interest.
Gustavo da Costa Bressan declares that he has no conflict of interest.
Juliana Lopes Rangel Fietto declares that she has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Rocha Torres Pavione, N., de Moraes, J.V.B., Ribeiro, I.C. et al. Heterologous expression and biochemical characterization of the recombinant nucleoside triphosphate diphosphohydrolase 2 (LbNTPDase2) from Leishmania (Viannia) braziliensis. Purinergic Signalling (2023). https://doi.org/10.1007/s11302-023-09980-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11302-023-09980-9