Abstract
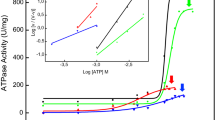
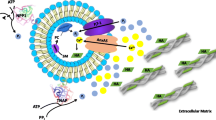
Matrix vesicles (MVs) are a special class of extracellular vesicles released by mineralizing cells during bone and tooth mineralization that initiate the precipitation of apatitic minerals by regulating the extracellular ratio between inorganic phosphate (Pi), a calcification promoter, and pyrophosphate (PPi), a calcification inhibitor. The Pi/PPi ratio is thought to be controlled by two ecto-phosphatases present on the outer leaflet of the MVs’ membrane: ectonucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) that produces PPi as well as Pi from ATP and tissue-nonspecific alkaline phosphatase (TNAP) that hydrolyzes both ATP and PPi to generate Pi. However, if and how these enzymes act in concert in MVs are still unclear. Herein, we investigated the role of NPP1 and TNAP in ATP hydrolysis during MV-mediated biomineralization using proteoliposomes as a biomimetic model for MVs. Proteoliposomes composed by 1,2-dipalmitoylphosphatidylcholine (DPPC) and harboring NPP1 alone, TNAP alone, or both together at different molar ratios (1:1, 10:1, and 1:10) were fabricated. After 48 h of incubation with ATP, TNAP-containing proteoliposomes consumed more ATP than NPP1-containing vesicles (270 and 210 nmol, respectively). Both types of vesicles comparatively formed ADP (205 and 201 nmol, respectively), while NPP1-containing vesicles hydrolyzed AMP less efficiently than TNAP-containing proteoliposomes (10 and 25 nmol, respectively). In vitro mineralization assays showed that in the presence of ATP, TNAP-harboring proteoliposomes mineralized through a sigmoidal single-step process, while NPP1-harboring vesicles displayed a two-step mineralization process. ATR-FTIR analyses showed that the minerals produced by TNAP-harboring proteoliposomes were structurally more similar to hydroxyapatite than those produced by NPP1-harboring vesicles. Our results with proteoliposomes indicate that the pyrophosphohydrolase function of NPP1 and the phosphohydrolase activity of TNAP act synergistically to produce a Pi/PPi ratio conducive to mineralization and the synergism is maximal when the two enzymes are present at equimolar concentrations. The significance of these findings for hypophosphatasia is discussed.





Similar content being viewed by others
Data availability
The dataset generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cruz MAE, Ferreira CR, Tovani CB et al (2020) Phosphatidylserine controls calcium phosphate nucleation and growth on lipid monolayers: a physicochemical understanding of matrix vesicle-driven biomineralization. J Struct Biol 212:107607. https://doi.org/10.1016/j.jsb.2020.107607
Bottini M, Mebarek S, Anderson KL et al (2018) Matrix vesicles from chondrocytes and osteoblasts: their biogenesis, properties, functions and biomimetic models. Biochim Biophys Acta - Gen Subj 1862:532–546. https://doi.org/10.1016/j.bbagen.2017.11.005
Von Euw S, Wang Y, Laurent G et al (2019) Bone mineral: new insights into its chemical composition. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-44620-6
Plaut JS, Strzelecka-Kiliszek A, Bozycki L et al (2019) Quantitative atomic force microscopy provides new insight into matrix vesicle mineralization. Arch Biochem Biophys 667:14–21. https://doi.org/10.1016/j.abb.2019.04.003
Allen MR, Burr DB (2019) Bone growth, modeling, and remodeling. Basic Appl Bone Biol 85–100 https://doi.org/10.1016/b978-0-12-813259-3.00005-1
Galea GL, Zein MR, Allen S, Francis-West P (2021) Making and shaping endochondral and intramembranous bones. Dev Dyn 250:414–449. https://doi.org/10.1002/dvdy.278
McKee MD, Buss DJ, Reznikov N (2022) Mineral tessellation in bone and the stenciling principle for extracellular matrix mineralization. J Struct Biol 214:107823. https://doi.org/10.1016/j.jsb.2021.107823
Buss DJ, Kröger R, McKee MD, Reznikov N (2022) Hierarchical organization of bone in three dimensions: a twist of twists. J Struct Biol X 6:0-9 https://doi.org/10.1016/j.yjsbx.2021.100057
Lowe M, Strauss AW, Alpers R et al (1990) Molecular cloning and expression of a cDNA encoding the membrane-associated rat intestinal alkaline phosphatase. Biochim Biophys Acta (BBA)/Protein Struct Mol 1037:170–177. https://doi.org/10.1016/0167-4838(90)90164-B
Le Du MH, Millán JL (2002) Structural evidence of functional divergence in human alkaline phosphatases. J Biol Chem 277:49808–49814. https://doi.org/10.1074/jbc.M207394200
Jansen S, Perrakis A, Ulens C et al (2012) Structure of NPP1, an ectonucleotide pyrophosphatase/phosphodiesterase involved in tissue calcification. Structure 20:1948–1959. https://doi.org/10.1016/j.str.2012.09.001
Lee SY, Müller CE (2017) Nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) and its inhibitors. Medchemcomm 8:823–840. https://doi.org/10.1039/c7md00015d
Huang R, Rosenbach M, Vaughn R et al (1994) Expression of the murine plasma cell nucleotide pyrophosphohydrolase PC-1 is shared by human liver, bone, and cartilage cells. Regulation of PC-1 expression in osteosarcoma cells by transforming growth factor-β. J Clin Invest 94:560–567. https://doi.org/10.1172/JCI117370
Szeri F, Niaziorimi F, Donnelly S, et al (2022) The mineralization regulator ANKH mediates cellular efflux of ATP, not pyrophosphate. J Bone Miner Res 0–3 https://doi.org/10.1002/jbmr.4528
Terkeltaub R, Rosenbach M, Fong F, Goding J (1994) Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteoblasts with plasma cell membrane glycoprotein–1. Arthritis Rheum 37:934–941. https://doi.org/10.1002/art.1780370624
Johnson K, Hashimoto S, Lotz M et al (2001) Up-regulated expression of the phosphodiesterase nucleotide pyrophosphatase family member PC-1 is a marker and pathogenic factor for knee meniscal cartilage matrix calcification. Arthritis Rheum 44:1071–1081. https://doi.org/10.1002/1529-0131(200105)44:5%3c1071::AID-ANR187%3e3.0.CO;2-3
Simão AMS, Yadav MC, Narisawa S et al (2010) Proteoliposomes harboring alkaline phosphatase and nucleotide pyrophosphatase as matrix vesicle biomimetics. J Biol Chem 285:7598–7609. https://doi.org/10.1074/jbc.M109.079830
Ciancaglini P, Yadav MC, Simão AMS et al (2010) Kinetic analysis of substrate utilization by native and TNAP-, NPP1-, or PHOSPHO1-deficient matrix vesicles. J Bone Miner Res 25:716–723. https://doi.org/10.1359/jbmr.091023
Bolean M, Simão AMS, Favarin BZ et al (2010) The effect of cholesterol on the reconstitution of alkaline phosphatase into liposomes. Biophys Chem 152:74–79. https://doi.org/10.1016/j.bpc.2010.08.002
Favarin BF, Andrade MAR, Bolean M et al (2017) Effect of the presence of cholesterol in the interfacial microenvironment on the modulation of the alkaline phosphatase activity during in vitro mineralization. Colloids Surfaces B Biointerfaces 155:466–476. https://doi.org/10.1016/j.colsurfb.2017.04.051
Goettsch C, Strzelecka-Kiliszek A, Bessueille L, et al (2020) TNAP as a therapeutic target for cardiovascular calcification: a discussion of its pleiotropic functions in the body. Cardiovasc Res 0–13https://doi.org/10.1093/cvr/cvaa299
Millán JL (2006) Alkaline phosphatases. Purinergic Signal 2:335–341. https://doi.org/10.1007/s11302-005-5435-6
Ciancaglini P, Simão AMS, Camolezi FL et al (2006) Contribution of matrix vesicles and alkaline phosphatase to ectopic bone formation. Brazilian J Med Biol Res 39:603–610. https://doi.org/10.1590/S0100-879X2006000500006
Ciancaglini P, Pizauro JM, Rezende AA et al (1990) Solubilization of membrane-bound matrix-induced alkaline phosphatase with polyoxyethylene 9-lauryl ether (polidocanol): purification and metalloenzyme properties. Int J Biochem 22:385–392. https://doi.org/10.1016/0020-711X(90)90141-O
Hartree EF (1972) Determination of protein: a modification of the lowry method that gives a linear photometric response. Anal Biochem 48:422–427. https://doi.org/10.1016/0003-2697(72)90094-2
Camolezi FL, Daghastanli KRP, Magalhães PP et al (2002) Construction of an alkaline phosphatase-liposome system: a tool for biomineralization study. Int J Biochem Cell Biol 34:1091–1101. https://doi.org/10.1016/S1357-2725(02)00029-8
Genge BR, Wu LNY, Wuthier RE (2007) Kinetic analysis of mineral formation during in vitro modeling of matrix vesicle mineralization: effect of annexin A5, phosphatidylserine, and type II collagen. Anal Biochem 367:159–166. https://doi.org/10.1016/j.ab.2007.04.029
Simão AMS, Bolean M, Favarin BZ et al (2019) Lipid microenvironment affects the ability of proteoliposomes harboring TNAP to induce mineralization without nucleators. J Bone Miner Metab 37:607–613. https://doi.org/10.1007/s00774-018-0962-8
Favarin BZ, Bolean M, Ramos AP et al (2020) Lipid composition modulates ATP hydrolysis and calcium phosphate mineral propagation by TNAP-harboring proteoliposomes. Arch Biochem Biophys 691:108482. https://doi.org/10.1016/j.abb.2020.108482
Garcia AF, Simão AMS, Bolean M et al (2015) Effects of GPI-anchored TNAP on the dynamic structure of model membranes 17:26295–26301. https://doi.org/10.1039/c5cp02377g
Ciancaglini P, Simão AMS, Bolean M et al (2012) Proteoliposomes in nanobiotechnology. Biophys Rev 4:67–81. https://doi.org/10.1007/s12551-011-0065-4
Yingchoncharoen P, Kalinowski DS, Richardson DR (2016) Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev 68:701–787. https://doi.org/10.1124/pr.115.012070
Ishikawa T, Wakamura M, Kondo S (1989) Surface characterization of calcium hydroxylapatite by Fourier transform infrared spectroscopy. Langmuir 5:140–144. https://doi.org/10.1021/la00085a025
Wuthier RE, Chin JE, Hale JE et al (1985) Isolation and characterization of calcium-accumulating matrix vesicles from chondrocytes of chicken epiphyseal growth plate cartilage in primary culture. J Biol Chem 260:15972–15979
Sauer GR, Wuthier RE (1988) Fourier transform infrared characterization of mineral phases formed during induction of mineralization by collagenase-released matrix vesicles in vitro. J Biol Chem 263:13718–13724. https://doi.org/10.1016/s0021-9258(18)68300-0
Bolean M, Simão AMS, Barioni MB et al (2017) Biophysical aspects of biomineralization. Biophys Rev 9:747–760. https://doi.org/10.1007/s12551-017-0315-1
Iqbal J, Lévesque SA, Sévigny J, Müller CE (2008) A highly sensitive CE-UV method with dynamic coating of silica-fused capillaries for monitoring of nucleotide pyrophosphatase/phosphodiesterase reactions. Electrophoresis 29:3685–3693. https://doi.org/10.1002/elps.200800013
Hoyle CHV, Knight GE, Burnstock G (1990) Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol 99:617–621. https://doi.org/10.1111/j.1476-5381.1990.tb12979.x
Wiedemar N, Hauser DA, Maser P (2020) 100 yeas of suramin. Antimicrob Agents Chemother 64:1–14. https://doi.org/10.1128/AAC.01168-19
Erceg I, Kontrec J, Strasser V et al (2022) Precipitation of calcium phosphates and calcium carbonates in the presence of differently charged liposomes. Minerals 2:1–20. https://doi.org/10.3390/min12020208
Bolean M, Simão AMS, Favarin BZ et al (2011) Thermodynamic properties and characterization of proteoliposomes rich in microdomains carrying alkaline phosphatase. Biophys Chem 158:111–118. https://doi.org/10.1016/j.bpc.2011.05.019
Song Y, Hahn HH, Hoffmann E (2002) Effects of solution conditions on the precipitation of phosphate for recovery: a thermodynamic evaluation. Chemosphere 48:1029–1034. https://doi.org/10.1016/S0045-6535(02)00183-2
Anderson HC, Harmey D, Camacho NP et al (2005) Sustained osteomalacia of long bones despite major improvement in other hypophosphatasia-related mineral deficits in tissue nonspecific alkaline phosphatase/nucleotide pyrophosphatase phosphodiesterase 1 double-deficient mice. Am J Pathol 166:1711–1720. https://doi.org/10.1016/S0002-9440(10)62481-9
Thouverey C, Bechkoff G, Pikula S, Buchet R (2009) Inorganic pyrophosphate as a regulator of hydroxyapatite or calcium pyrophosphate dihydrate mineral deposition by matrix vesicles. Osteoarthr Cartil 17:64–72. https://doi.org/10.1016/j.joca.2008.05.020
Eller P, Hochegger K, Feuchtner GM et al (2008) Impact of ENPP1 genotype on arterial calcification in patients with end-stage renal failure. Nephrol Dial Transplant 23:321–327. https://doi.org/10.1093/ndt/gfm566
Millán JL, Whyte MP (2016) Alkaline phosphatase and hypophosphatasia. Calcif Tissue Int 98:398–416. https://doi.org/10.1007/s00223-015-0079-1
Nitschke Y, Rutsch F (2012) Genetics in arterial calcification: lessons learned from rare diseases. Trends Cardiovasc Med 22:145–149. https://doi.org/10.1016/j.tcm.2012.07.011
Whyte MP, Rockman-Greenberg C, Ozono K et al (2016) Asfotase alfa treatment improves survival for perinatal and infantile hypophosphatasia. J Clin Endocrinol Metab 101:334–342. https://doi.org/10.1210/jc.2015-3462
Yadav MC, Bottini M, Cory E et al (2016) Skeletal mineralization deficits and impaired biogenesis and function of chondrocyte-derived matrix vesicles in phospho1 -/- and phospho1/P i t1 double-knockout mice. J Bone Miner Res 31:1275–1286. https://doi.org/10.1002/jbmr.2790
Shimada BK, Pomozi V, Zoll J et al (2021) ABCC6, pyrophosphate and ectopic calcification: therapeutic solutions. Int J Mol Sci 22:1–29. https://doi.org/10.3390/ijms22094555
Sheen CR, Kuss P, Narisawa S et al (2015) Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J Bone Miner Res 30:824–836. https://doi.org/10.1002/jbmr.2420
Zhang Y, Brown MA, Peach C et al (2007) Investigation of the role of ENPP1 and TNAP genes in chondrocalcinosis. Rheumatology 46:586–589. https://doi.org/10.1093/rheumatology/kel338
Bennett RM, Lehr JR, McCarty DJ (1975) Factors affecting the solubility of calcium pyrophosphate dihydrate crystals. J Clin Invest 56:1571–1579. https://doi.org/10.1172/JCI108239
Hessle L, Johnson KA, Anderson HC et al (2002) Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A 99:9445–9449. https://doi.org/10.1073/pnas.142063399
Anderson HC, Sipe JB, Hessle L et al (2004) Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol 164:841–847. https://doi.org/10.1016/S0002-9440(10)63172-0
Yadav MC, Simão AMS, Narisawa S et al (2011) Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification. J Bone Miner Res 26:286–297. https://doi.org/10.1002/jbmr.195
Acknowledgements
We wish to dedicate this study to the memory of Professor Geoffrey Burnstock, whose pioneering work led to the development of an entire field of research on purinergic signaling in physiology and pathophysiology. We also would like to thank Mrs. Mercia V. Carlos from the Chemistry Department of FFCLRP-USP for assistance with the HPLC analysis.
Funding
We would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2017/20846–2; 2019/08568–2; 2019/25054–2; 2021/13140-1), Coordination for the Improvement of Higher Education Personnel (CAPES Finance Code 001, 1738449, 88887.320304/2019‐00, 88887.368020/2019–00), National Council for Scientific and Technological Development (CNPq, 304021/2017‐2 and 305426/2021–4), PVE-Print CAPES-USP 2020–88887.569449/2020–00, and Bando Visiting Professor – Prot. n. 0058868 12/12/2021—Tor Vergata Università degli Studi di Roma. M. Bolean received CAPES grant and LHSA received FAPESP grant. APR and PC are CNPq researchers.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
The corresponding author is available to send the documentation of compliance with ethical standards if requested.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andrilli, L.H.S., Sebinelli, H.G., Favarin, B.Z. et al. NPP1 and TNAP hydrolyze ATP synergistically during biomineralization. Purinergic Signalling 19, 353–366 (2023). https://doi.org/10.1007/s11302-022-09882-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-022-09882-2