Abstract
Chronic cough is the most common complaint in respiratory clinics. Most of them have identifiable causes and some may respond to common disease-modifying therapies. However, there are many patients whose cough lacks effective aetiologically targeted treatments or remains unexplained after thorough assessments, which have been described as refractory chronic cough. Current treatments for refractory chronic cough are limited and often accompanied by intolerable side effects such as sedation. In recent years, various in-depth researches into the pathogenesis of chronic cough have led to an explosion in the development of drugs for the treatment of refractory chronic cough. There has been considerable progress in the underlying mechanisms of chronic cough targeting ATP, and ongoing or completed clinical studies have confirmed the promising antitussive efficacy of P2X3 antagonists for refractory cough. Herein, we review the foundation on which ATP target was developed as potential antitussive medications and provide an update on current clinical progresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
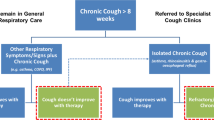
Cough is an important physiological protective reflex to avoid aspiration into the airways and to maintain airway patency. However, in some individuals excessive, dry or minimally productive cough becomes problematic leading to decrement in quality of life [1]. The commonly adopted definition of chronic cough (CC) in adults is a cough lasting for at least 8 weeks [2]. Following the current cough guidelines, the majority of patients with chronic cough can have identifiable causes and some may respond to common disease-modifying therapies. However, there are many patients whose cough lacks effective aetiologically targeted treatments or remains unexplained after thorough assessments. A variety of terms have been used to describe this condition which is referred to (synonymously) as refractory chronic cough (RCC) or unexplained chronic cough (UCC) in recent literature [3, 4].
RCC has been reported as a common clinical problem, with a worldwide prevalence of approximately 10% [5]. In specialist cough clinics, the prevalence may be up to 59.1% [6]. These patients often share a common feature in that their troublesome cough is often triggered by levels of stimuli such as perfumes and change in temperature which ordinarily would not cause cough in healthy people. This is characterized as allotussia to innocuous stimuli, abnormal sensations in the throat (laryngeal paresthesia), and increased response to tussive stimuli (hypertussia). Collectively, this is known as cough hypersensitivity syndrome (CHS) [7, 8]. This common clinical presentation is thought be due to dysregulation of neuronal pathways arising from the airways. Dysregulation may include both peripheral and central pathways. This latter provides the foundation for the importance of neuromodulators in treating RCC, such as currently used gabapentin and baclofen [9]. At present, it is unclear whether these medications are likely to have a non-specific antitussive effect in RCC by inhibiting the sensitized central cough pathways. Their efficacy is poor being only around 50% even in uncontrolled studies, and with prominent drug-related adverse events such as sedation [10, 11]. A single agent, low-dose morphine, has been shown in a placebo-controlled RCT to be efficacious in about a third of patients with RCC [12].
A significant body of evidence has indicated the peripheral mechanisms are of importance in cough hypersensitivity. Inhalation challenge studies have demonstrated increased cough reflex cough sensitivity from peripheral stimulation. Inhalation of capsaicin, cinnamaldehyde, allyl-isothiocyanate indicates that peripheral sensor receptors such as transient receptor potential vanilloid 1 (TRPV1), transient receptor potential vanilloid 4 (TRPV4), and transient receptor potential ankyrin 1 (TRPA1) receptors have an important role in upregulating the cough sensitivity. However, their antagonists did not show efficacy in RCC clinical trials [13,14,15,16,17]. RCC patients are thus bereft of effective treatments and undergo significant physical, psychological, and socio-economic stress, with consequent serious negative impacts on the quality of life (QoL) [4, 18]. The treatment of RCC is currently a therapeutic black hole and there is an urgent need for safe, effective, non-sedating medications for the treatment of this common condition.
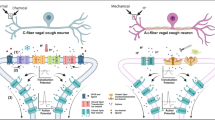
Recently, knowledge of neural pathways in RCC has been advanced through focus on adenosine triphosphate (ATP) activating purinergic P2X3 receptors (Fig. 1). Great progress has been made in the exploration of P2X3 antagonists as potential antitussive medications. Herein, we review the foundation on which ATP target was developed as potential antitussive medications and provide an update on current clinical progresses.
Current understanding of the neural processes in the cough reflex. Tussive stimuli from various sources can increase the calcium influx, leading to ATP release from the open pannexin-1 channel. This in turn activates the P2X3 and P2X2/3 receptors on sensory neurones within the airway mucosa. Other ion channels (TRPV1, TRPA1, TRPV4, TRPM8) on nociceptor terminals originating from jugular or nodose ganglia are activated by irritants or inflammatory reactions. These processes combine to produce an action potential, which is carried along the vagus nerve to cough centre (nTS and Pa5) and onwards to the central nervous system to regulate cough reflex. This is a gross oversimplification of an extremely complex neural pathway. The precise mechanism of cough still remains to be elucidated, particularly the mechanism producing the hypersensitization seen in patients with chronic cough. Other pathways and receptor systems are likely to be revealed by future work. ATP: adenosine triphosphate; nTS: nucleus of the solitary tract; Pa5: paratrigeminal nucleus; Ca2+: calcium; Na+: sodium; TRPV: transient receptor potential vanilloid; TRPA: transient receptor potential ankyrin; TRPM: transient receptor potential melastatin; NaV: voltage-gated sodium channel
ATP as a key modulator of the cough reflex
ATP was long known as an intracellular energy source involved in metabolic processes of all cells. When released extracellularly, it is hydrolyzed to AMP and then adenosine by extracellular nucleotidases. In 1972, Geoffery Burnstock proposed that ATP and related nucleotides could be neurotransmitters co-released with noradrenaline, which subsequently acted on non-adrenergic and non-cholinergic nerves [19, 20]. This new concept was not widely accepted initially, but gradually evidence accumulated suggesting a role for the purinergic signalling. The role of ATP was confirmed 20 years later when the first P2X subtype was cloned [21]. In recent years, many studies have focused on the role of extracellular signalling by ATP released in response to cell damage—the so-called alarmin concept. The demonstration of P2X receptors on the peripheral afferent nerves supports the role of ATP as a short-term signalling molecule in disorders in multiple different systems, such as visceral pain, bladder incontinence, hypertension, and chronic cough [22, 23].
That the release of extracellular ATP plays a major role in RCC has been now well established. After release from non-neuronal cells such as the injured airway epithelium and immune cells by cell lysis or through pannexin channels on the plasma membrane, ATP can stimulate afferent sensory nerves and release other proinflammatory cytokines driving further inflammation [24,25,26]. This can induce acute peripheral sensitization, as revealed by the acute cough response to ATP inhalation challenge in healthy volunteers (HV). Patients with RCC exhibited a greater degree of response to ATP inhalation [27, 28]. ATP also showed a more potent effect than AMP on inducing cough and bronchoconstriction in asthmatic patients [29, 30]. With aerosolized ATP, more dyspnea, cough, and throat irritation can also be observed in smokers and patients with COPD, when compared to healthy subjects [31]. Furthermore, in bronchoalveolar lavage fluid (BALF) of chronic smokers and ex-smokers with chronic obstructive pulmonary disease (COPD), elevated ATP concentrations were detected, indicating ATP could contribute to the symptoms and inflammation in the pathogenesis of some chronic respiratory diseases [32]. Similar findings can be seen in asthmatic humans [33]. Given that cough is a major symptom of airway disease, this suggests the likelihood that ATP could directly or indirectly enhance the cough reflex in diverse respiratory conditions.
Some animal studies help to verify the plausibility of the involvement of ATP in cough. However, the effect of ATP appears to manifest a significant species difference. Generally, most of the peripheral sensory fibres innervating the respiratory tract are originated from the vagus, and relay through two distinct ganglia referred to as the nodose and the jugular ganglia. The peripheral stimuli activate C fibres which respond to a wide range of chemical and mechanical stimuli, signalling to respiratory central neural circuits [1]. A rapid ATP bolus administration into right atrium or pulmonary artery of a canine model could only stimulate the afferent capsaicin-sensitive vagal C fibre terminals and trigger a vagal reflex [34]. In mice, ATP can activate both capsaicin-sensitive and capsaicin-insensitive C fibres [35]. Given the difficulty in mimicking cough in models such as mice or rats [36], most early cough challenge studies were conducted using guinea pigs. In guinea pigs, capsaicin-insensitive nodose ganglia neurons, which terminate intrapulmonarily, can be activated by ATP (some specialized Aδ fibres, which are commonly named cough receptors, terminate in the larger airways and do not have action potential firing in respond to ATP); but the jugular ganglia neurons, which terminate in the larynx, trachea, and mainstem bronchi, are all ATP-insensitive [37,38,39]. The non-hydrolyzable form of ATP, α, β-methylene ATP, was found to have a direct and receptor-dependent effect on the rapidly adapting receptors (RARs, also called Aβ fibres); however, it failed to evoke cough in conscious guinea pigs [37]. Later, Kamei and his colleagues confirmed that the extracellular ATP, by itself, did not elicit cough, with an acute guinea pig cough model in vivo, but caused increased coughing in response to citric acid. They also found that the ATP-induced cough sensitivity to citric acid was not changed by the desensitization of C fibres, but capsaicin-induced coughs were reduced. Based on the fact that citric acid stimulates both vagal C fibres and RARs, whereas capsaicin appears to only act on C fibres, they proposed the likelihood that RARs were involved in the pathway of ATP action in cough [40].
Cough is usually evoked by stimuli from larynx, trachea, and large airways, which have the jugular chemosensitive terminals and the specialized nodose Aδ fibre terminals (cough receptors). It is puzzling that all these terminals are ATP-insensitive. In recent years, jugular airway nociceptors, rather than nodose ganglia, were thought to play a critical role in the induction and sensitization of cough in non-human species, which may in part be in agreement with the weak tussigenic feature of ATP by itself [41, 42]. However, this does not conflict with the theory that ATP sensitizes the irritated cough sensor and exacerbates cough.
Taken together, these in vivo animal studies give confusing pictures of the cough reflex pathways in different animal species and give little insight into the role of ATP in RCC.
Strength of rationale for P2X3 purinergic receptor inhibition
Purinergic receptors are divided into 2 classes—A receptors (also termed P1R), whose ligand is adenosine, and P2 receptors (P2R, which primarily recognize nucleotides usually AMP and ATP). The P2 receptors contain two further subtypes: G protein–coupled receptors (P2Y, subunits (functional human receptors) are numbered 1, 2, 4, 6, 11, 12, 13, and 14) and ligand-gated ion channels (P2X, homotrimers or heterotrimers of subunits numbered 1–7) [43, 44]. The expression of purinergic receptors and ectonucleotidases varies in different tissues or cells under physiological and pathophysiological situations.
Once ATP is released from the cells under pathological conditions, it will act on P2 receptors as a local mediator in an autocrine or paracrine manner. The releases of these and other distress signals are collectively known as alarmins. Currently, there is a growing understanding of purinergic signalling in almost every system. Since ATP can enhance cough reflex in pulmonary diseases, it is reasonable to assume that the blockade of ATP receptors on vagal fibres could modulate cough hypersensitivity. In vivo studies pointed to the expression of functional P2X receptors on the nodoses neurons projecting C-fibres to the lungs of guinea pigs and canines [34, 39]. In 2005, Kamei and his colleagues found ATP-induced enhanced cough reactivity to citric acid in guinea pigs could be abolished by TNP-ATP, an antagonist of P2X1-4 [40]. Later, in 2006, they reported that the combination of TNP-ATP and reactive blue 2 (a P2Y antagonist) could completely eliminate the histamine-induced increased cough reactivity to citric acid in guinea pigs [45]. Homotrimeric P2X3 receptors (e.g. with three P2X3 subunits) and heterotrimeric P2X2/3 receptors (e.g., with two P2X3 subunits and one P2X2 subunit), which are expressed in both peripheral and central terminals of the vagus, are the most investigated subunits of P2X receptors [46, 47]. P2X3 receptors were first cloned in 1995 and were demonstrated to be located on small nociceptive sensory neurons in dorsal root ganglia (DRG) with lectin IB4 in 1998. Geoffery Burnstock proposed a P2X3 purinergic hypothesis for the initiation of pain in 1996 [48,49,50]. Later, the P2X3 knockout mice confirmed this receptor’s importance in the field of sensory processing, nociceptive signalling, and hollow organ biology [51,52,53,54]. Given the similar hollow organ biological features in the respiratory tract, and the similarity of cough to the physiology of neuropathic pain, RCC was described as a distinct clinical entity and termed neuropathic cough in the last decade [55,56,57]. Many studies provided the mechanistic evidence for targeting P2X3 as a promising antitussive therapeutic indication. In an ex vivo study, ATP-mediated nodose C fibre activation was found to be inhibited with the P2X2/3 and P2X3 purinoceptors antagonists [58]. The extracellular patch-clamp electrophysiology and single cell RT-PCR analysis revealed the expression of P2X2/3 heteromeric receptors on C fibres derived from nodose ganglion neurons, whereas the jugular neurons primarily expressed homomeric P2X3 receptors [47]. Puzzlingly, in these studies, only heteromeric P2X2/3 receptors were functional, i.e. have a sustained current when activated. In contrast, P2X2 and P2X3 gene knock out mice confirmed that both receptors play an important role in nociceptions [59]. Moreover, the evidence in the preclinical guinea pig cough model that BLU-5937 (a selective P2X3 antagonist) reduced the histamine-enhanced cough reflex to citric acid, and that aerosolized DT-0111 (a selective and effective P2X2/3 antagonist) inhibited ATP-induced bronchoconstriction and cough, intimated the potential for potent antitussive activity of P2X3 antagonist [60, 61]. Taken together, these evidence suggests that P2X2 and P2X3 receptors play different roles dependent on the species and the pathophysiological stimuli provoking their activation.
P2X receptor antagonists in clinic trials for RCC
P2X receptor antagonists previously widely used in the preclinical studies, such as pyridoxal phosphate-6-azo (benzene-2,4-disulphonic acid) (PPADS), suramin, the dye reactive blue 2, and 2′,3′-O-(2,4,6-trinitrophenyl) ATP (TNP-ATP), have limited potency, selectivity, stability, and poor pharmacokinetics, and have therefore not progressed to clinic trial programmes [62, 63]. A- 317,491 is the first identified competitive and reversible P2X3 antagonist with good systemic bioavailability; however, its water solubility and oral bioavailability are not good [64]. Alternative chemical antagonists with drug-like characteristics such as good oral bioavailability, slow clearance, little blood–brain barrier permeability, and high safety margin have been developed. In the last 7–8 years, there had been an explosion in the clinical application of several novel P2X3 receptor antagonists in RCC (Table 1).
Gefapixant
Gefapixant (previously known as AF-219 and MK-7264) was named after Geoffery Burnstock and is the first in class P2X3 and P2X2/3 receptor-selective antagonist. This molecule, as with all other antagonist in development, is reversible and is an allosteric (non-competitive) antagonist. It was firstly developed by Roche and subsequently licenced to Afferent who undertook the initial successful clinical studies. Merck then purchased the rights for over one billion dollars. It has a good pharmacokinetic profile and low potential to cause a clinically relevant drug-drug interaction [82,83,84,85]. A range of clinical studies have been undertaken leading to two phase III trials which have recently been successfully completed. They confirmed its antitussive efficacy and regulatory approval is expected shortly for the treatment of RCC in practice [86].
The phase I study examined the pharmacokinetics, safety, and tolerability of gefapixant by profiling a very large range dose and exposure levels in HV and subsequently confirmed its high oral bioavailability and resistance to metabolic degradation [62]. The initial proof-of-concept (POC) phase II study of AF-219 suggested its promising efficacy in RCC [65]. In this randomized, double-blind, placebo-controlled, crossover study in 24 RCC patients, gefapixant produced significant reductions in objective cough frequency. However, the high dose administered (600 mg twice daily) produced marked taste disturbances (hypogeusia or dysgeusia), which caused withdrawal in 25% subjects. In another double-blind, randomized, 2-period, crossover phase II study [28], which was conducted on 24 CC patients and 12 HV, a lower single-dose gefapixant 100 mg inhibited ATP-evoked cough in CC and HV, as well as distilled water–evoked cough in CC, but had no effect on capsaicin or citric acid challenge. Median cough frequency was reduced by 42% with gefapixant over placebo in CC subjects. This suggests the underlying role of TRPV4/ATP-mediated P2X3 receptor activation in the pathophysiology of chronic cough as a peripheral target. The preservation of the protective irritant-induced cough demonstrated in this study is an important safety signal and subsequent larger studies have failed to demonstrate any excess of aspiration pneumonia with P2X3 antagonist. Taste-related issues were again the most common adverse events, during which dysgeusia was reported in 75% HV and 67% CC.
The notable taste adverse events are thought to be due to the relatively poor selectivity of gefapixant for the P2X3 receptor over the P2X2/3 heterotrimer. This may lead to unmasking effects in clinical trials and inhibit medication compliance. Therefore, the exploration of the optimal cough relief of P2X3 antagonist with a diminished or even eliminated side effect on taste was the objective in the subsequence phase II studies. Two crossover-designed randomized dose-escalation studies (study 1: 50–200 mg, twice daily; study 2: 7.5–50 mg, twice daily) were reported in the European Respiratory Journal in 2020, and allowed the calculation of optimal dosing [66]. Reduction of the awake cough frequency was maximal at dose ≥ 30 mg, two times daily, which was far lower than the dose in the POC. Taste disturbances were also dose-dependent. On the basis of these findings, a randomized, double-blinded, controlled, parallel-group, phase IIb study was performed, evaluating the efficacy of gefapixant at one of three doses of 7.5 mg, 20 mg, or 50 mg, twice daily, over 12 weeks [67]. With the dose of 50 mg, geometric mean of awake cough frequency was reduced by 37%, relative to placebo, and with marked improvements in cough-related assessments; taste disturbances occurred in 81% patients although at a much-reduced severity.
Two global, parallel, double-blind, randomized placebo-controlled phase III trials (COUGH-1 and COUGH-2) were designed [68] and completed in March 2020. Subjects were administrated either placebo, gefapixant 15 mg or 45 mg, twice daily, in a ratio of 1:1:1. Study period differed, 12 weeks for COUGH-1 (extension periods of 40 weeks) and 24 weeks for COUGH-2 (extension periods of 28 weeks). A total of 2044 RCC patients were recruited, in COUGH-1 (n = 730) and COUGH-2 (n = 1314). Gefapixant at the dose of 45 mg reduced 24-h cough frequency by 18.5% in COUGH-1 and 14.6% in COUGH-2, relative to placebo. The placebo response was considerably higher in these phase III studies with a reduction of 24-h cough count greater than 50% compared to the approximate 30% seen in phase II studies. Taste disturbance remained to be the most common adverse events, with the incidence of 59.3% in COUGH-1 and 68.9% in COUGH-2, with most being tolerated and reversing after cessation of treatment. However, 15 mg of gefapixant, two times daily, had no significant efficacy compared to placebo, which presumably swamped any treatment effect. Thus, 45 mg of gefapixant was finally proven to be an effective antitussive option for these RCC patients who had a mean duration of cough greater than 10 years. The reports of COUGH-1 and COUGH-2 were published in The Lancet [87].
In a trial of idiopathic pulmonary fibrosis cough [88], 50 mg of gefapixant, two times daily, demonstrated a poor efficacy in awake cough frequency and a similar incidence of efficacy-unrelated taste disturbance (78.7%) [89]. This may suggest the heterogeneity in the underlying etiology in disease-specific cough and RCC although there were a number of methodological problems in this study.
Because of the allosteric nature of P2X3 antagonists, their roles in mediating taste signalling or cough hypersensitivity has not been well established as yet [90]. P2X2/3 heterotrimeric receptors were speculated to play a dominant role in taste signalling with evidence that the chorda tympani and glossopharyngeal nerves failed to response to all taste qualities in P2X2 and P2X3 double knockout mice, but did not exhibit such severe taste disturbance in P2X2 or P2X3 single knockout mice [91, 92]. Given that gefapixant demonstrated approximately threefold low degree of selectivity for P2X3 homotrimers over P2X2/3 heterotrimers [57, 84], a new generation of antagonists with higher selectivity to P2X3 are under clinical development.
Eliapixant (BAY-1817080)
A novel, highly selective (confirmed by patch clamp studies) P2X3 receptor antagonist, eliapixant, was developed by Bayer (BAY-1817080)[82, 93]. In vivo studies showed its potential efficacy in nerve hypersensitization [93]. Eliapixant has been reported for its good tolerability in HV after single and multiple dosing [70]. Promising efficacy for RCC was shown in a phase IIa study [71]. In this randomized, placebo-controlled, double-blinded, crossover study, eliapixant was administered twice daily in two treatment periods: 2 weeks of placebo followed by 1 week of 10 mg and escalating doses of 50, 200, and 750 mg, each for 1 week. Forty RCC patients were assessed with the change in objective 24-h cough frequency as the primary endpoint. Doses ≥ 50 mg demonstrated a 15% reduction in cough frequency compared with placebo, with a lower incidence of taste-related side effects of 10–21% (all mild). Recently, Bayer reported the most current encouraging results of the international placebo-controlled, randomized, double-blind, parallel group, phase IIb dose-finding study of eliapixant in RCC at the European Respiratory Society (ERS) International Congress 2021 [72]. A total of 310 participants were administrated orally with either 25, 75, or 150 mg of eliapixant or placebo tablets, twice daily, for 12 weeks. Seventy-five-milligram dose of eliapixant twice daily could reduce objective cough frequency by 27% over placebo, with the majority of side effects considered mild or moderate. Taste-related side effects were reported in 24% of patients with the highest test dose of 150 mg, which were markedly less under lower doses. Taken together, these studies confirmed a lower incidence of taste-related issues with eliapixant at effective therapeutic doses. That eliapixant has demonstrated efficacy in RCC confirms the hypothesis that the P2X3 antagonists, as a class, have an important role in the treatment of this previously intractable condition. Despite the promising clinical trial results with eliapixant clinical trial, development has been suspended by Bayer because of a risk of hepatotoxicity (elevated transaminases) seen in a small number of patients exposed to the 150-mg dosage.
BLU-5937
BLU-5937 is another potent non-competitive antagonist stereoselective to P2X3 homotrimeric receptor developed by Bellus Health. It exhibited excellent drug-like characteristics in preclinical studies and showed potential efficacy with limited or no taste disturbance in animal cough model [60]. The randomized, double-blind, placebo-controlled phase I study recruited 90 HV to assess the safety, tolerability, and pharmacokinetic profile of BLU-5937 [73]. During the administration of single ascending doses (50, 100, 200, 400, 800, 1200 mg) or doses of 100, 200, 400 mg, twice daily, for 7 days, BLU-5937 presented excellent pharmacokinetic and safety/tolerability profiles, with only one case of mild, transient, and sporadic taste alteration at the anticipated therapeutic doses (500–100 mg). Recently, the top-line results from the POC phase IIa randomized, double-blinded, placebo-controlled, two-period, crossover, dose-escalation study (the RELIEF trial) of BLU-5937 in RCC patients have been reported at the American Thoracic Society International Conference 2021 [74]. Sixty-nine RCC participants were randomized to 16-day treatment (25, 50, 100, and 200 mg, twice daily) or matching placebo, with dose escalation every 4 days, then were crossed over after a 10–14-day washout. This trial was terminated early due to COVID-19 limitations and failed to reach significant reductions in the awake cough counts in the intent-to-treat population. However, significant reductions were observed in a pre-planned sub-group analysis of patients with higher baseline cough frequency: awake cough frequencies at baseline of ≥ 20 coughs/h (− 23.8%, − 19.1%, and − 27.3% at 25, 50, and 200 mg, twice daily, respectively, over placebo) or ≥ 32 coughs/h (− 29.0%, − 28.8%, − 27.1%, and − 32.1% at 25, 50, 100, and 200 mg, twice daily, respectively, over placebo). According to data presented by Bellus Health [75], taste-related side effects were infrequent at all dose levels, which were 6.5%, 9.8%, 10%, and 8.6% at 25, 50, 100, and 200 mg, respectively, versus 4.9% with placebo, and were mostly mild in nature. No patients reported complete taste loss. Higher cough counts were assumed to be the best available clinical indicator of cough hypersensitization via the P2X3 pathway; however, conflicting results have been seen in other studies and this may represent a statistical artefact akin to regression to the mean. These data moved forward BLU-5937 to an adaptive phase IIb trial for RCC patients with higher cough counts [76, 94]. This is a multi-centre, randomized, double-blind, parallel arm dose-finding study (the SOOTHE trial), and included a placebo run-in period and stratification by baseline cough frequency. A total of 240 patients with a baseline awake cough frequency ≥ 25 coughs/h were randomized to the three active treatment arms of BLU-5937 (12.5, 50, and 200 mg, twice daily, 1:1:1:1) or placebo for 4 weeks after a single-blind run-in period. An exploratory population of participants (n = 60) with baseline awake cough frequencies between 10 and 25 coughs/h will be randomized to placebo and BLU-5937 200 mg twice daily treatment arms (1:1). The primary endpoint is the reduction in objective 24-h cough frequency versus placebo. In December 2021, Bellus Health announced the positive top-line results of this study [77]. Significant placebo-adjusted improvement of 34% was observed in 24-h cough frequency (the primary efficacy endpoint) at 50 mg and 200 mg BID doses with a few taste-related adverse events (≤ 6.5%).
Sivopixant (S-600918)
Sivopixant (also called S-600918), firstly reported by Shionogi, is a newly developed antagonist with favourable pharmacokinetic profiles and higher selectivity to P2X3 over P2X2/3 trimeric homomer [95]. Its promising antitussive efficacy with limited taste-related side effects for RCC has been demonstrated in a POC phase IIa, randomized, double-blind, placebo-controlled, crossover, multicentre study [78]. In this study, 31 RCC patients were randomized to oral sivopixant 150 mg or placebo once daily for 2 weeks, then crossed over for another 2 weeks after a 2–3-week washout. The placebo-adjusted cough reductions in the average hourly objective coughs in day-time (primary outcome) and in 24 h (secondary outcome) were − 31.6% (p = 0.0546) and − 30.9% (p = 0.0386), respectively, accompanied with the significant improvement in health-related quality of life measured with Leicester Cough Questionnaire but not in Visual Analogue Scoring. Only 2 cases of mild taste disturbance (6.5%) were observed. The authors attributed the lack of statistical significance in primary outcome to the insufficient sample size. However, in the subsequent phase IIb dose selection study (ClinicalTrials.gov identifier: NCT04110054), which was conducted in 372 RCC subjects assigned to 50, 150, or 300 mg of oral S-600918 or matched placebo for 4 weeks, the statistically significant placebo-adjusted change in 24-h cough frequency (primary efficacy endpoint) was not met at any dose. Incidence of taste-related side effects was dose dependent (2.0%, 13.6%, 33.0% at 50, 150, or 300 mg, respectively, versus 2.9% with placebo) [79, 80]. These top-line primary data were reported at Shionogi R&D Day, September 29th, 2021. Next steps are under consideration.
In this study, as with the difference between gefapixant phase II and the phase III studies, a larger placebo effect was seen. In both cases, earlier recruitment had been limited to specialized centres experienced in dealing with RCC patients who have usually undergone multiple failed treatment trials. Placebo response may be greater in treatment-naïve patients and centres. This is an important consideration for the design of future multicentre phase 3 studies where correct patient selection will be vital in demonstrating efficacy over placebo response.
Filapixant (BAY-1902607)
Filapixant, also named BAY-1902607, is another new P2X3 antagonist with high selectivity, developed by Bayer. Currently, it has been investigated in a phase I/II trial in RCC in Netherlands and UK (p.o.) (NCT03535168) (EudraCT2018-000,129–29) [96]. No recent reports of development were identified for phase I development in cough (in volunteers) in Germany (p.o., tablet). Phase I study has been reported as completed on ClinicalTrials.gov, but data have not yet been posted or published. Primary data of efficacy and side effects from phase II POC study have been presented at the ERS International Congress 2020 [81]. Twenty-three RCC patients were randomized to ascending doses of BAY1902607 (20, 80, 150, or 250 mg, twice daily, 4 days each) or placebo in 2-way crossover. The significant decreases in objective 24-h coughs per hour and cough severity were observed at doses ≥ 80 mg. Taste-related side effects were mild-to-moderate and dose-dependent (4–57% with BAY-1902607 versus 12% with placebo). Despite the high selectivity to P2X3, BAY-1902607 had fairly high impact on taste. The complete data is awaited.
Other antagonists for P2X3 receptors
DT-0111 (Aspirex™), being developed by Danmir Therapeutic, LLC., is a novel, small, water-soluble molecule that acts as a selective antagonist at P2X2/3 receptors. In vitro and in vivo POC studies have demonstrated its potential to be an inhalation drug candidate for ATP-related pulmonary diseases such as chronic obstructive pulmonary disease (COPD) and chronic cough [61]. In addition, Obrecht et al. identified aurintricarboxylic acid (ATA) as a nanomolar-potency allosteric antagonist of P2X3 and P2X1 receptors with weak inhibition to P2X2/3 receptors [97]. However, the clinical evidence for their antitussive effects has not yet been studied.
Other purinergic receptor targets with promising antitussive effect
P2X4 receptor seems to be an interesting target in modulating cough reflex. P2X4 and P2X4/P2X6 heterotrimers are moderately expressed in the lung [98]. In guinea pigs, ATP-induced enhancement of the number of citric acid–induced coughs was ameliorated by exposure to TNP-ATP (an antagonist of P2X1–4 receptors), but without response to PPADS (an antagonist of P2X1,2,3,5,7 receptors, but not of P2X4); thus, P2X4 receptor was thought to be involved in the ATP-induced enhancement of the cough reflex sensitivity [40]. In vitro and in vivo studies provided evidence that P2X4 is the predominant subunit of P2X expressed in secretory airway epithelial cells and its overexpression could be seen in conditions of chronic inflammation, mucous metaplasia, and hyperplasia [99]. Moreover, PSB-15417, a potent, brain-permeable allosteric P2X4 receptor antagonist for human, rat, and mouse, showed high efficacy in rat models of neuropathic pain, which shares the similar underlying mechanisms with RCC [44, 100]. Another P2X4 receptor antagonist, NC-2600, is currently in clinical trials for chronic neuropathic pain in Japan [101]. P2X7 receptor is also closely associated with neuropathic pain, which has been proved in the spinal cord levels and amygdala [102, 103]. However, the correlation between P2X7 and RCC is lack of sufficient direct evidence, except for the role of P2X7 in mediating ATP efflux [104].
In terms of P2Y receptors, which are expressed in almost all epithelial cells and responsible for fluid control and electrolyte transport, the submits of P2Y2, P2Y4, P2Y6, and P2Y14 are relatively strongly expressed in epithelial and glandular cells of lungs to regulate the physiological functions of respiratory system [98, 105, 106]. The upregulation of P2Y4 and P2Y6 have been detected in allergic bronchospasm, which may lead to an increased production of endogenous ATP via exocytosis following P2Y4 activation, or intensify the inflammatory response via raising inflammatory factors following P2Y6 activation [107,108,109]. Moreover, combined with TNP-ATP, exposure to reactive blue 2, a P2Y receptor antagonist, could completely reduce histamine-induced increased coughs to citric acid; thus, P2Y receptors were assumed to play partial role in ATP-induced cough hyperreactivity [45]. However, further study to confirm this assumption is warranted.
In the long history of antitussive drug development, there has been many promising targets which have demonstrated efficacy in animal models. A good example is that the TRP receptors and the TRPV1 receptor agonist capsaicin have been long used in cough challenge models. Highly specific TRPV1 antagonist was developed which showed excellent efficacy in capsaicin challenge models demonstrating target engagement. However, these agents have showed no efficacy in RCC [13, 14] and indeed a TRPV4 antagonist was actually showed to provoke cough in RCC [17]. Caution is therefore required in interpreting the results of the preclinical studies of other purinergic receptor targets.
Taken together, given the breakthroughs in P2X3 receptors and taste-related limitations in patients, new drugs with antagonistic activity for other purinergic receptor targets with possible antitussive effect should be taken into consideration.
Conclusions and perspectives
RCC is a complicated neurobiological process with marked heterogeneity, and which involves multiple peripheral and central neural pathways [110]. Efficacy of the available medications varies from patient to patient, and it seems unlikely to expect a complete elimination of cough symptoms with a single agent. Currently, none of the clinical trials, whether P2X3 antagonists or other neuromodulators, has achieved this goal. Future clinical management of chronic cough will almost certainly require polymodal therapeutic approaches. The antitussive effects of P2X3 antagonists, which suppress the ATP-mediated sensitization of nociceptors, provide potent evidence for ATP-P2X3 pathway in regulating the pathological cough reflex. However, a third of patients fail to respond. Thus, ATP seems unlikely to act as a common mediator for all tussive stimuli which sensitize nerves. Indeed, it is still unclear as to how ATP drives the chronic cough hypersensitization and which sensory neuron pathway is responsible for transmitting the noxious sensation [27, 111]. The combination of further understanding of the neural mechanisms and analysis of clinical variables in RCC patients is essential to identify cough phenotypes in the future. More work is required to identify further therapeutic targets to alleviate this common and chronic disease.
Data availability
Not applicable.
References
Singh N, Driessen AK, McGovern AE et al (2020) Peripheral and central mechanisms of cough hypersensitivity. J Thorac Dis 12:5179–5193. https://doi.org/10.21037/jtd-2020-icc-007
Morice AH, Millqvist E, Bieksiene K et al (2020) ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 55:1901136. https://doi.org/10.1183/13993003.01136-2019
Gibson P, Wang G, McGarvey L et al (2016) Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest 149:27–44. https://doi.org/10.1378/chest.15-1496
Morice A, Dicpinigaitis P, McGarvey L, et al (2021). Chronic cough: new insights and future prospects. Eur Respir Rev 30https://doi.org/10.1183/16000617.0127-2021
Song W, Chang Y, Faruqi S et al (2015) The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 45:1479–1481. https://doi.org/10.1183/09031936.00218714
Al-Sheklly B, Satia I, Badri H et al (2018) P5 Prevalence of refractory chronic cough in a tertiary cough clinic. Thorax 73:A98–A98. https://doi.org/10.1136/thorax-2018-212555.163
Morice AH, Millqvist E, Belvisi MG et al (2014) Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 44:1132–1148. https://doi.org/10.1183/09031936.00218613
Song W-J, Morice AH (2017) Cough hypersensitivity syndrome: a few more steps forward. Allergy Asthma Immunol Res 9:394–402. https://doi.org/10.4168/aair.2017.9.5.394
Farrell MJ, Mazzone SB (2019) Are neural pathways processing airway inputs sensitized in patients with cough hypersensitivity? Pulm Pharmacol Ther 57:101806. https://doi.org/10.1016/j.pupt.2019.101806
Dong R, Xu X, Yu L et al (2019) Randomised clinical trial: gabapentin vs baclofen in the treatment of suspected refractory gastro-oesophageal reflux-induced chronic cough. Aliment Pharmacol Ther 49:714–722. https://doi.org/10.1111/apt.15169
Zhang M, Zhu Y, Dong R et al (2020) Gabapentin versus baclofen for treatment of refractory gastroesophageal reflux-induced chronic cough. J Thorac Dis 12:5243–5250. https://doi.org/10.21037/jtd-2020-icc-002
Morice AH, Menon MS, Mulrennan SA et al (2007) Opiate therapy in chronic cough. Am J Respir Crit Care Med 175:312–315. https://doi.org/10.1164/rccm.200607-892OC
Khalid S, Murdoch R, Newlands A et al (2014) Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J Allergy Clin Immunol 134:56-62.e54. https://doi.org/10.1016/j.jaci.2014.01.038
Belvisi MG, Birrell MA, Wortley MA et al (2017) XEN-D0501, a novel transient receptor potential vanilloid 1 antagonist, does not reduce cough in patients with refractory cough. Am J Respir Crit Care Med 196:1255–1263. https://doi.org/10.1164/rccm.201704-0769OC
Smith J, Allman D, Badri H et al (2020) The neurokinin-1 receptor antagonist orvepitant is a novel antitussive therapy for chronic refractory cough: results from a phase 2 pilot study (VOLCANO-1). Chest 157:111–118. https://doi.org/10.1016/j.chest.2019.08.001
Agency. EM (2016. Date last accessed: April 15, 2019. ). Clinical trial results: a phase 2a, multi-centre, randomised, double-blind, parallel group, placebo-controlled study to evaluate efficacy, safety and tolerability of inhaled GRC 17536, administered for 4 weeks, in patients with refractory chronic cough. EU Clinical Trials Registry. www.clinicaltrialsregister.eu/ctr-search/trial/2013-002728-17/results.
Ludbrook VJ, Hanrott KE, Kreindler JL, et al (2021). Adaptive study design to assess effect of TRPV4 inhibition in patients with chronic cough. ERJ Open Res 7https://doi.org/10.1183/23120541.00269-2021
French CL, Crawford SL, Bova C et al (2017) Change in psychological, physiological, and situational factors in adults after treatment of chronic cough. Chest 152:547–562. https://doi.org/10.1016/j.chest.2017.06.024
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24:509–581
Schwiebert EM, Zsembery A (2003) Extracellular ATP as a signaling molecule for epithelial cells. Biochimica et Biophysica Acta (BBA) - Biomembranes 1615:7–32. https://doi.org/10.1016/s0005-2736(03)00210-4
Valera S, Hussy N, Evans R et al (1994) A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature 371:516–519. https://doi.org/10.1038/371516a0
Burnstock G (2017) Purinergic signalling: therapeutic developments. Front Pharmacol 8:661. https://doi.org/10.3389/fphar.2017.00661
Burnstock G (2020) Introduction to purinergic signaling. Methods Mol Biol 2041:1–15. https://doi.org/10.1007/978-1-4939-9717-6_1
Dahl G (2015). ATP release through pannexon channels. Philos Trans R Soc Lond B Biol Sci 370https://doi.org/10.1098/rstb.2014.0191
Kouzaki H, Iijima K, Kobayashi T et al (2011) The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol 186:4375–4387. https://doi.org/10.4049/jimmunol.1003020
Burnstock G, Brouns I, Adriaensen D et al (2012) Purinergic signaling in the airways. Pharmacol Rev 64:834–868. https://doi.org/10.1124/pr.111.005389
Fowles HE, Rowland T, Wright C et al (2017) Tussive challenge with ATP and AMP: does it reveal cough hypersensitivity? Eur Respir J 49:1601452. https://doi.org/10.1183/13993003.01452-2016
Morice AH, Kitt MM, Ford AP, et al (2019). The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J 54https://doi.org/10.1183/13993003.00439-2019
Basoglu OK, Pelleg A, Kharitonov SA et al (2017) Contrasting effects of ATP and adenosine on capsaicin challenge in asthmatic patients. Pulm Pharmacol Ther 45:13–18. https://doi.org/10.1016/j.pupt.2017.04.004
Basoglu OK, Pelleg A, Essilfie-Quaye S et al (2005) Effects of aerosolized adenosine 5’-triphosphate vs adenosine 5’-monophosphate on dyspnea and airway caliber in healthy nonsmokers and patients with asthma. Chest 128:1905–1909. https://doi.org/10.1378/chest.128.4.1905
Basoglu OK, Barnes PJ, Kharitonov SA et al (2015) Effects of aerosolized adenosine 5’-triphosphate in smokers and patients with COPD. Chest 148:430–435. https://doi.org/10.1378/chest.14-2285
Lommatzsch M, Cicko S, Muller T et al (2010) Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181:928–934. https://doi.org/10.1164/rccm.200910-1506OC
Idzko M, Hammad H, van Nimwegen M et al (2007) Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 13:913–919. https://doi.org/10.1038/nm1617
Pelleg A, Hurt C (1996) Mechanism of action of ATP on canine pulmonary vagal C fibre nerve terminals. J Physiol 490:265–275. https://doi.org/10.1113/jphysiol.1996.sp021142
Kollarik M, Dinh QT, Fischer A et al (2003) Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol 551:869–879. https://doi.org/10.1113/jphysiol.2003.042028
Mazzone SB, Undem BJ (2016) Vagal afferent innervation of the airways in health and disease. Physiol Rev 96:975–1024. https://doi.org/10.1152/physrev.00039.2015
Canning BJ, Mazzone SB, Meeker SN et al (2004) Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557:543–558. https://doi.org/10.1113/jphysiol.2003.057885
Canning BJ, Mori N, Mazzone SB (2006) Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol 152:223–242. https://doi.org/10.1016/j.resp.2006.03.001
Undem BJ, Chuaychoo B, Lee MG et al (2004) Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol 556:905–917. https://doi.org/10.1113/jphysiol.2003.060079
Kamei J, Takahashi Y, Yoshikawa Y et al (2005) Involvement of P2X receptor subtypes in ATP-induced enhancement of the cough reflex sensitivity. Eur J Pharmacol 528:158–161. https://doi.org/10.1016/j.ejphar.2005.10.030
Moe AAK, McGovern AE, Mazzone SB (2021) Jugular vagal ganglia neurons and airway nociception: a target for treating chronic cough. Int J Biochem Cell Biol 135:105981. https://doi.org/10.1016/j.biocel.2021.105981
Chou Y-L, Mori N, Canning BJ (2018) Opposing effects of bronchopulmonary C-fiber subtypes on cough in guinea pigs. Am J Physiol Regul Integr Comp Physiol 314:R489–R498. https://doi.org/10.1152/ajpregu.00313.2017
Jacobson KA, AP IJ, Müller CE (2021) Medicinal chemistry of P2 and adenosine receptors: common scaffolds adapted for multiple targets. Biochem Pharmacol 187:114311. https://doi.org/10.1016/j.bcp.2020.114311
Muller CE, Namasivayam V (2021). Recommended tool compounds and drugs for blocking P2X and P2Y receptors. Purinergic Signalhttps://doi.org/10.1007/s11302-021-09813-7
Kamei J, Takahashi Y (2006) Involvement of ionotropic purinergic receptors in the histamine-induced enhancement of the cough reflex sensitivity in guinea pigs. Eur J Pharmacol 547:160–164. https://doi.org/10.1016/j.ejphar.2006.07.034
Gever JR, Cockayne DA, Dillon MP et al (2006) Pharmacology of P2X channels. Pflugers Arch 452:513–537. https://doi.org/10.1007/s00424-006-0070-9
Kwong K, Kollarik M, Nassenstein C et al (2008) P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol 295:L858-865. https://doi.org/10.1152/ajplung.90360.2008
Chen C-C, Akopian AN, Sivilottit L et al (1995) A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377:428–431. https://doi.org/10.1038/377428a0
Bradbury EJ, Burnstock G, McMahon SB (1998) The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci 12:256–268. https://doi.org/10.1006/mcne.1998.0719
Burnstock G (1996) A unifying purinergic hypothesis for the initiation of pain. Lancet 347:1604–1605. https://doi.org/10.1016/s0140-6736(96)91082-x
Cockayne D, Hamilton S, Zhu Q et al (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407:1011–1015. https://doi.org/10.1038/35039519
Vulchanova L, Riedl M, Shuster S et al (1997) Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology 36:1229–1242. https://doi.org/10.1016/s0028-3908(97)00126-3
Bian X, Ren J, DeVries M et al (2003) Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol 551:309–322. https://doi.org/10.1113/jphysiol.2003.044172
Ochoa-Cortes F, Linan-Rico A, Jacobson KA et al (2014) Potential for developing purinergic drugs for gastrointestinal diseases. Inflamm Bowel Dis 20:1259–1287. https://doi.org/10.1097/MIB.0000000000000047
Michalowski A, Haines A, Shaparin N et al (2021) Transcutaneous electrical nerve stimulation as a treatment for neuropathic cough: a tolerability and feasibility study. Neurol Ther. https://doi.org/10.1007/s40120-021-00255-2
Cohen SM, Misono S (2013) Use of specific neuromodulators in the treatment of chronic, idiopathic cough: a systematic review. Otolaryngology-head and neck surgery 148:374–382. https://doi.org/10.1177/0194599812471817
Ford AP, Undem BJ (2013) The therapeutic promise of ATP antagonism at P2X3 receptors in respiratory and urological disorders. Front Cell Neurosci 7:267. https://doi.org/10.3389/fncel.2013.00267
Weigand LA, Ford AP, Undem BJ (2012) A role for ATP in bronchoconstriction-induced activation of guinea pig vagal intrapulmonary C-fibres. J Physiol 590:4109–4120. https://doi.org/10.1113/jphysiol.2012.233460
Cockayne DA, Dunn PM, Zhong Y et al (2005) P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol 567:621–639. https://doi.org/10.1113/jphysiol.2005.088435
Garceau D, Chauret N (2019) BLU-5937: a selective P2X3 antagonist with potent anti-tussive effect and no taste alteration. Pulm Pharmacol Ther 56:56–62. https://doi.org/10.1016/j.pupt.2019.03.007
Pelleg A, Xu F, Zhuang J et al (2019) DT-0111: a novel drug-candidate for the treatment of COPD and chronic cough. Ther Adv Respir Dis 13:1753466619877960. https://doi.org/10.1177/1753466619877960
Ford AP, Dillon MP, Kitt MM et al (2021) The discovery and development of gefapixant. Auton Neurosci 235:102859. https://doi.org/10.1016/j.autneu.2021.102859
Jacobson K, Jarvis M, Williams M (2002) Purine and pyrimidine (P2) receptors as drug targets. J Med Chem 45:4057–4093. https://doi.org/10.1021/jm020046y
Jarvis MF (2021) Geoffery Burnstock’s influence on the evolution of P2X3 receptor pharmacology. Purinergic Signal 17:33–39. https://doi.org/10.1007/s11302-020-09744-9
Abdulqawi R, Dockry R, Holt K et al (2015) P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 385:1198–1205. https://doi.org/10.1016/s0140-6736(14)61255-1
Smith JA, Kitt MM, Butera P, et al (2020). Gefapixant in two randomised dose-escalation studies in chronic cough. Eur Respir J, 55https://doi.org/10.1183/13993003.01615-2019
Smith JA, Kitt MM, Morice AH et al (2020) Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med 8:775–785. https://doi.org/10.1016/s2213-2600(19)30471-0
Muccino DR, Morice AH, Birring SS, et al (2020). Design and rationale of two phase 3 randomised controlled trials (COUGH-1 and COUGH-2) of gefapixant, a P2X3 receptor antagonist, in refractory or unexplained chronic cough. ERJ Open Res, 6https://doi.org/10.1183/23120541.00284-2020
McGarvey LP, Birring SS, Morice AH et al (2021) Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, placebocontrolled phase 3 trials. In press, Lancet
Christian F, Klaus F, Isabella G et al (2021) Safety, pharmacodynamics, and pharmacokinetics of P2X3 receptor antagonist eliapixant (BAY 1817080) in healthy subjects double-blind, randomized study. In press, Clinical Pharmacokinetics
Morice A, Smith JA, McGarvey L, et al (2021). Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: a randomised, placebo-controlled, crossover phase 2a study. Eur Respir J https://doi.org/10.1183/13993003.04240-2020
Lorcan McGarvey, Alyn H Morice, Jaclyn Smith, et al (2021). Late breaking abstract - efficacy and safety of eliapixant in refractory chronic cough: results of the PAGANINI 12-week, randomized, placebo-controlled phase 2b study. Eur Respir J, PA562. https://doi.org/10.1183/13993003.congress-2021.PA562.
Garceau D, Chauret N, Harvey L (2020) BLU-5937, A highly selective P2X3 homotrimeric receptor antagonist, exhibits excellent pharmacokinetic and safety profile including improved taste safety profile in healthy subjects. Lung 198:38–39. https://doi.org/10.1007/s00408-020-00328-3
Smith J, Morice AH, Birring SS, et al (2021). Improvements in cough frequency over 24 hours with BLU-5937, a selective P2X3 antagonist, in patient subgroups defined by baseline awake cough frequencies. Am J Respiratory Critical Care Med 203https://doi.org/10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A1019
Businesswire A Berkshire Hathaway Company [Internet]. Laval (QC); 2020 [cited 2021 Dec 30]. Available from: https://www.businesswire.com/news/home/20200706005125/en/BELLUS-Health-Announces-Topline-Results-Phase-2.
Bonuccelli CM, Smith J, Birring SS, et al (2021). Design of soothe, a phase 2b dose finding study with blu-5937, a selective p2x3 antagonist, in refractory chronic cough. Am J Respiratory Critical Care Med 203https://doi.org/10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A2356
LAVAL, Quebec--(BUSINESS WIRE)--Dec. 13, 2021-- BELLUS Health Inc. BELLUS Health Announces Positive Topline BELLUS Health announces positive topline results from its phase 2b SOOTHE Trial of BLU-5937 for the treatment of refractory chronic cough. Available from: https://ir.bellushealth.com/news-releases/news-release-details/bellus-health-announces-positive-topline-results-its-phase-2b.
Niimi A, Saito J, Kamei T, et al (2021). Randomised trial of the P2X(3) receptor antagonist sivopixant for refractory chronic cough. Eur Respir Jhttps://doi.org/10.1183/13993003.00725-2021
Ishihara H, Hida H, Machida M et al (2020) Design of phase 2b randomised controlled trial of S-600918, P2X3 receptor antagonist for refractory chronic cough. Eur Respir J 56:2271. https://doi.org/10.1183/13993003.congress-2020.2271
SHIONOGI & Co., Ltd. [cited 2021 Dec 30]. Available from: https://www.shionogi.com/content/dam/shionogi/global/investors/ir-library/presentation/2021/e_210929_3(3).pdf.
Friedrich C, Francke K, Birring SS, et al (2020). Safety and efficacy of P2X3 antagonist BAY 1902607 in refractory chronic cough. European Respiratory J 56https://doi.org/10.1183/13993003.congress-2020.4566
Spinaci A, Buccioni M, Dal Ben D et al (2021) P2X3 receptor ligands: structural features and potential therapeutic applications. Front Pharmacol 12:653561. https://doi.org/10.3389/fphar.2021.653561
McCrea JB, Hussain A, Ma B, et al (2021). Assessment of pharmacokinetic interaction between gefapixant (MK-7264), a P2X3 receptor antagonist, and the OATP1B1 drug transporter substrate pitavastatin. Clin Pharmacol Drug Devhttps://doi.org/10.1002/cpdd.1047
Richards D, Gever JR, Ford AP et al (2019) Action of MK-7264 (gefapixant) at human P2X3 and P2X2/3 receptors and in vivo efficacy in models of sensitisation. Br J Pharmacol 176:2279–2291. https://doi.org/10.1111/bph.14677
Nussbaum JC, Hussain A, Ma B et al (2022) Assessment of the effect of pyrimethamine, a potent inhibitor of multidrug and toxin extrusion protein 1/2K, on the pharmacokinetics of gefapixant (MK-7264), a P2X3 receptor antagonist. Clin Pharmacol Drug Dev 11:123–128. https://doi.org/10.1002/cpdd.988
Abu-Zaid A, Aljaili AK, Althaqib A et al (2021) Safety and efficacy of gefapixant, a novel drug for the treatment of chronic cough: a systematic review and meta-analysis of randomized controlled trials. Ann Thorac Med 16:127–140. https://doi.org/10.4103/atm.ATM_417_20
McGarvey LP, Birring SS, Morice AH et al (2022) Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. The Lancet 399:909–923. https://doi.org/10.1016/S0140-6736(21)02348-5
Vigeland CL, Hughes AH, Horton MR (2017) Etiology and treatment of cough in idiopathic pulmonary fibrosis. Respir Med 123:98–104. https://doi.org/10.1016/j.rmed.2016.12.016
Martinez FJ, Afzal AS, Smith JA, et al (2021). Treatment of persistent cough in subjects with idiopathic pulmonary fibrosis (IPF) with gefapixant, a P2X3 antagonist, in a randomized, placebo-controlled clinical trial. Pulm Therhttps://doi.org/10.1007/s41030-021-00162-9
Dicpinigaitis PV, McGarvey LP, Canning BJ (2020) P2X3-receptor antagonists as potential antitussives: summary of current clinical trials in chronic cough. Lung 198:609–616. https://doi.org/10.1007/s00408-020-00377-8
Kinnamon S, Finger T (2021). The role of ATP and purinergic receptors in taste signaling. Handb Exp Pharmacolhttps://doi.org/10.1007/164_2021_518
Finger T, Danilova V, Barrows J et al (2005) ATP signaling is crucial for communication from taste buds to gustatory nerves. Science (New York, NY) 310:1495–1499. https://doi.org/10.1126/science.1118435
Davenport AJ, Neagoe I, Brauer N et al (2021) Eliapixant is a selective P2X3 receptor antagonist for the treatment of disorders associated with hypersensitive nerve fibers. Sci Rep 11:19877. https://doi.org/10.1038/s41598-021-99177-0
Grabczak EM, Dabrowska M, Birring SS et al (2020) Looking ahead to novel therapies for chronic cough. Part 1 - peripheral sensory nerve targeted treatments. Expert Rev Respir Med 14:1217–1233. https://doi.org/10.1080/17476348.2020.1811686
Kai H, Horiguchi T, Kameyma T et al (2021) Discovery of clinical candidate Sivopixant (S-600918): lead optimization of dioxotriazine derivatives as selective P2X3 receptor antagonists. Bioorg Med Chem Lett 52:128384. https://doi.org/10.1016/j.bmcl.2021.128384
Mazzone SB, McGarvey L (2021) Mechanisms and rationale for targeted therapies in refractory and unexplained chronic cough. Clin Pharmacol Ther 109:619–636. https://doi.org/10.1002/cpt.2003
Obrecht A, Urban N, Schaefer M et al (2019) Identification of aurintricarboxylic acid as a potent allosteric antagonist of P2X1 and P2X3 receptors. Neuropharmacology 158:107749. https://doi.org/10.1016/j.neuropharm.2019.107749
Zhang M, Wang S, Yu L et al (2020) The role of ATP in cough hypersensitivity syndrome: new targets for treatment. J Thorac Dis 12:2781–2790. https://doi.org/10.21037/jtd-20-cough-001
Winkelmann VE, Thompson KE, Neuland K et al (2019) Inflammation-induced upregulation of P2X4 expression augments mucin secretion in airway epithelia. Am J Physiol Lung Cell Mol Physiol 316:L58–L70. https://doi.org/10.1152/ajplung.00157.2018
Teixeira JM, Dos Santos GG, Neves AF et al (2019) Diabetes-induced neuropathic mechanical hyperalgesia depends on P2X4 receptor activation in dorsal root ganglia. Neuroscience 398:158–170. https://doi.org/10.1016/j.neuroscience.2018.12.003
Jacobson KA, Giancotti LA, Lauro F et al (2020) Treatment of chronic neuropathic pain: purine receptor modulation. Pain 161:1425–1441. https://doi.org/10.1097/j.pain.0000000000001857
Hu X, Liu Y, Wu J et al (2020) Inhibition of P2X7R in the amygdala ameliorates symptoms of neuropathic pain after spared nerve injury in rats. Brain Behav Immun 88:507–514. https://doi.org/10.1016/j.bbi.2020.04.030
Song J, Ying Y, Wang W et al (2018) The role of P2X7R/ERK signaling in dorsal root ganglia satellite glial cells in the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR). Brain Behav Immun 69:180–189. https://doi.org/10.1016/j.bbi.2017.11.011
Suadicani SO, Brosnan CF, Scemes E (2006) P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci 26:1378–1385. https://doi.org/10.1523/JNEUROSCI.3902-05.2006
Burnstock G (2007) Purine and pyrimidine receptors. Cell Mol Life Sci 64:1471–1483. https://doi.org/10.1007/s00018-007-6497-0
Rafehi M, Muller CE (2018) Tools and drugs for uracil nucleotide-activated P2Y receptors. Pharmacol Ther 190:24–80. https://doi.org/10.1016/j.pharmthera.2018.04.002
Chavez J, Vargas MH, Rebollar-Ayala DC et al (2013) Inhibition of extracellular nucleotides hydrolysis intensifies the allergic bronchospasm. A novel protective role of ectonucleotidases. Allergy 68:462–471. https://doi.org/10.1111/all.12113
Chavez J, Vargas MH, Martinez-Zuniga J et al (2019) Allergic sensitization increases the amount of extracellular ATP hydrolyzed by guinea pig leukocytes. Purinergic Signal 15:69–76. https://doi.org/10.1007/s11302-019-09644-7
Iv K (2021) Molecular pharmacology of P2Y receptor subtypes. Biochem Pharmacol 187:114361. https://doi.org/10.1016/j.bcp.2020.114361
Mazzone SB, Farrell MJ (2019) Heterogeneity of cough neurobiology: clinical implications. Pulm Pharmacol Ther 55:62–66. https://doi.org/10.1016/j.pupt.2019.02.002
Turner RD, Birring SS (2019). Chronic cough: ATP, afferent pathways and hypersensitivity. Eur Respir J 54https://doi.org/10.1183/13993003.00889-2019
Funding
AHM has received grant funding and advisory board fees from Merck, Shionogi, Bellus, Bayer, and Nerre.
Author information
Authors and Affiliations
Contributions
The literature search was performed by Mengru Zhang and Dominic L. Sykes. The first draft of the manuscript was written by Mengru Zhang. Prof. Alyn H. Morice had the idea for the work and was in charge of the review and correction of the manuscript. All authors critically revised the work and approved it for publication.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
Mengru Zhang declares that she has no relevant financial or non-financial conflict of interest; Dominic L. Sykes declares that he has no relevant financial or non-financial conflict of interest; Laura R. Sadofsky declares that she has no relevant financial or non-financial conflict of interest; Alyn H. Morice declares that he has received grant funding and advisory board fees from Merck, Shionogi, Bellus, Bayer, and Nerre.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, M., Sykes, D.L., Sadofsky, L.R. et al. ATP, an attractive target for the treatment of refractory chronic cough. Purinergic Signalling 18, 289–305 (2022). https://doi.org/10.1007/s11302-022-09877-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-022-09877-z