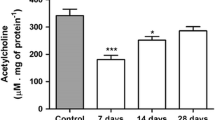
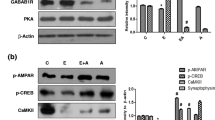
Abstract
Gallic acid (GA) is a secondary metabolite found in plants. It has the ability to cross the blood-brain barrier and, through scavenging properties, has a protective effect in a brain insult model. Alcohol metabolism generates reactive oxygen species (ROS); thus, alcohol abuse has a deleterious effect on the brain. The zebrafish is a vertebrate often used for screening toxic substances and in acute ethanol exposure models. The aim of this study was to evaluate whether GA pretreatment (24 h) prevents the changes induced by acute ethanol exposure (1 h) in the purinergic signaling pathway in the zebrafish brain via degradation of extracellular nucleotides and oxidative stress. The nucleotide cascade promoted by the nucleoside triphosphate diphosphohydrolase (NTPDase) and 5′-nucleotidase was assessed by quantifying nucleotide metabolism. The effect of GA alone at 5 and 10 mg L−1 did not change the nucleotide levels. Pretreatment with 10 mg L−1 GA prevented an ethanol-induced increase in ATP and ADP levels. No significant difference was found between the AMP levels of the two pretreatment groups. Pretreatment with 10 mg L−1 GA prevented ethanol-enhanced lipid peroxidation and dichlorodihydrofluorescein (DCFH) levels. The higher GA concentration was also shown to positively modulate against ethanol-induced effects on superoxide dismutase (SOD), but not on catalase (CAT). This study demonstrated that GA prevents the inhibitory effect of ethanol on NTPDase activity and oxidative stress parameters, thus consequently modulating nucleotide levels that may contribute to the possible protective effects induced by alcohol and purinergic signaling.




Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. The raw data are available from Eduardo Pacheco Rico upon reasonable request.
Abbreviations
- DCF:
-
2′,7′-dihydrodichlorofluorescein diacetate
- ATP:
-
Adenosine 5′ triphosphate
- CAT:
-
Catalase
- CNS:
-
Central nervous system
- DTNB:
-
5,5-dithio-bis-(2-nitrobenzoic acid
- NTPDase:
-
Nucleoside triphosphate diphosphohydrolase
- SOD:
-
Superoxide dismutase
- TBA-RS:
-
Thiobarbituric acid-reactive species
References
Badhani B, Sharma N, Kakkar R (2015) Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv 5:27540–27557. https://doi.org/10.1039/c5ra01911g
Grundhöfer P, Niemetz R, Schilling G, Gross GG (2001) Biosynthesis and subcellular distribution of hydrolyzable tannins. Phytochemistry 57:915–927. https://doi.org/10.1016/S0031-9422(01)00099-1
Komolafe K, Olaleye TM, Omotuyi OI, Boligon AA, Athayde ML, Akindahunsi AA et al (2014) In vitro antioxidant activity and effect of Parkia biglobosa bark extract on mitochondrial redox status. JAMS J Acupunct Meridian Stud 7:202–210. https://doi.org/10.1016/j.jams.2013.08.003
Giftson JS, Jayanthi S, Nalini N (2010) Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1,2-dimethyl hydrazine induced rat colon carcinogenesis. Investig New Drugs 28:251–259. https://doi.org/10.1007/s10637-009-9241-9
Hajipour S, Sarkaki A, Farbood Y, Eidi A, Mortazavi P, Valizadeh Z (2016) Effect of gallic acid on dementia type of Alzheimer disease in rats: electrophysiological and histological studies. Basic. Clin Neurosci 7:97–106. https://doi.org/10.15412/j.bcn.03070203
Ye Q, Ye L, Xu X, Huang B, Zhang X, Zhu Y et al (2012) Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1α signaling pathway. BMC Complement Altern Med 12:1. https://doi.org/10.1186/1472-6882-12-82
Mansouri MT, Farbood Y, Sameri MJ, Sarkaki A, Naghizadeh B, Rafeirad M (2013) Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem 138:1028–1033. https://doi.org/10.1016/j.foodchem.2012.11.022
Pervin M, Unno K, Nakagawa A, Takahashi Y, Iguchi K, Yamamoto H et al (2017) Blood brain barrier permeability of (−)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem Biophys Reports 9:180–186. https://doi.org/10.1016/j.bbrep.2016.12.012
Farbood Y, Sarkaki A, Hashemi S, Mansouri MT, Dianat M (2013) The effects of gallic acid on pain and memory following transient global ischemia/reperfusion in Wistar rats. Avicenna J Phytomedicine 3:329–340
Choi YT, Jung CH, Lee SR, Bae JH, Baek WK, Suh MH et al (2001) The green tea polyphenol (-)-epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci 70:603–614. https://doi.org/10.1016/S0024-3205(01)01438-2
WHO. Global status report on alcohol and health (2018) Geneva: World Health Organization; 2018. https://doi.org/10.1037/cou0000248.
Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M (2005) Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci 8:339–345. https://doi.org/10.1038/nn1398
Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, Valenzuela CF, Hansel C (2008) Alcohol impairs long-term depression at the cerebellar parallel fiber-Purkinje cell synapse. J Neurophysiol 100:3167–3174. https://doi.org/10.1152/jn.90384.2008
Gerlai R, Lahav M, Guo S, Rosenthal A (2000) Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav 67:773–782. https://doi.org/10.1016/s0091-3057(00)00422-6
Tran S, Gerlai R (2014) Recent advances with a novel model organism: alcohol tolerance and sensitization in zebrafish (Danio rerio). Prog Neuro-Psychopharmacol Biol Psychiatry 55:87–93. https://doi.org/10.1016/j.pnpbp.2014.02.008
Rico EP, Rosemberg DB, Senger MR, de Bem AM, Dias RD, Souto AA, Bogo MR, Bonan CD (2008) Ethanol and acetaldehyde alter NTPDase and 5'-nucleotidase from zebrafish brain membranes. Neurochem Int 52:290–296. https://doi.org/10.1016/j.neuint.2007.06.034
Zenki KC, Mussulini BH, Rico EP, de Oliveira DL, Rosemberg DB (2014) Effects of ethanol and acetaldehyde in zebrafish brain structures: an in vitro approach on glutamate uptake and on toxicity-related parameters. Toxicol in Vitro 28:822–828. https://doi.org/10.1016/j.tiv.2014.03.008
Zimmermann H (2011) Purinergic signaling in neural development. Semin Cell Dev Biol 22:194–204. https://doi.org/10.1016/j.semcdb.2011.02.007
Agteresch HJ, Dagnelie PC, van den Berg JW, Wilson J (1999) Adenosine triphosphate: established and potential clinical applica tions. Drugs 58:211–232. https://doi.org/10.2165/00003495-199958020-00002 2
Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:231–304. https://doi.org/10.1016/S0074-7696(04)40002-3
Ricatti MJ, Battista AG, Zorrilla Zubilete M, Faillace MP (2011) Purinergic signals regulate daily S-phase cell activity in the ciliary marginal zone of the zebrafish retina. J Biol Rhythm 26:107–117. https://doi.org/10.1177/0748730410395528
Appelbaum L, Skariah G, Mourrain P, Mignot E (2007) Comparative expression of p2x receptors and ecto-nucleoside triphosphate diphosphohydrolase 3 in hypocretin and sensory neurons in zebrafish. Brain Res 1174:66–75. https://doi.org/10.1016/j.brainres.2007.06.103
Low SE, Kuwada JY, Hume RI (2008) Amino acid variations resulting in functional and nonfunctional zebrafish P2X1 and P2X5.1 receptors. Purinergic Signal 4:383–392. https://doi.org/10.1007/s11302-008-9124-0
Rico EP, Senger MR, Fauth MDG, Dias RD, Bogo MR, Bonan CD (2003) ATP and ADP hydrolysis in brain membranes of zebrafish (Danio rerio). Life Sci 73:2071–2082. https://doi.org/10.1016/S0024-3205(03)00596-4
Rosemberg DB, Rico EP, Langoni AS, Spinelli JT, Pereira TC, Dias RD et al (2010) NTPDase family in zebrafish: nucleotide hydrolysis, molecular identification and gene expression profiles in brain, liver and heart. Comp Biochem Physiol B Biochem Mol Biol 155:230–240. https://doi.org/10.1016/j.cbpb.2009.11.005
Senger MR, Rico EP, Dias RD, Bogo MR, Bonan CD (2004) Ecto-5′-nucleotidase activity in brain membranes of zebrafish (Danio rerio). Comp Biochem Physiol B Biochem Mol Biol 139:203–207. https://doi.org/10.1016/j.cbpc.2004.07.011
Wyatt C, Bartoszek EM, Yaksi E (2015) Methods for studying the zebrafish brain: past, present and future. Eur J Neurosci 42:1746–1763. https://doi.org/10.1111/ejn.12932
Rosemberg DB, da Rocha RF, Rico EP, Zanotto-Filho A, Dias RD, Bogo MR, Bonan CD, Moreira JC, Klamt F, Souza DO (2010) Taurine prevents enhancement of acetylcholinesterase activity induced by acute ethanol exposure and decreases the level of markers of oxidative stress in zebrafish brain. Neuroscience. 171:683–692. https://doi.org/10.1016/j.neuroscience.2010.09.030
Torres CA, Mendes NV, Baldin SL, Bernardo HT, Vieira KM, Scussel R, de Bem SG, Silveira PCL, Machado-de-Ávila RA, Rico EP (2021) Cotreatment of small gold nanoparticles protects against the increase in cerebral acetylcholinesterase activity and oxidative stress induced by acute ethanol exposure in the zebrafish. Neuroscience. 457:41–50. https://doi.org/10.1016/j.neuroscience.2021.01.011
Agostini JF, Santo GD, Baldin SL, Bernardo HT, de Farias ACS, Rico EP, Wanderley AG (2021) gallic acid reverses neurochemical changes induced by prolonged ethanol exposure in the zebrafish brain. Neuroscience. 455:251–262. https://doi.org/10.1016/j.neuroscience.2020.11.040
Westerfield M (2000) The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). University of Oregon Press, Eugene
Dlugos CA, Rabin RA (2003) Ethanol effects on three strains of zebrafish: model system for genetic investigations. Pharmacol Bio chem Behav 74:471–480. https://doi.org/10.1016/s0091-3057(02)01026-2
Barnes JM, Murphy PA, Kirkham D, Henley J (1993) Interaction of guanine nucleotides with [3H] kainate and 6-[3H]cyano-7-nitroquinoxaline-2,3-dione binding in goldfish brain. J Neurochem 61:1685–1691. https://doi.org/10.1111/j.1471-4159.1993.tb09804.x
Altenhofen S, Dreher Nabinger D, Carneiro T, Pereira B, Leite CE, Reis Bogo M et al (2018) Manganese(II) chloride alters nucleotide and nucleoside catabolism in zebrafish (Danio rerio) adult brain. Mol Neurobiol 55:3866–3874. https://doi.org/10.1007/s12035-017-0601-8
Voelter W, Zech K, Arnold P, Ludwig G (1980) Determination of selected pyrimidines, purines and their metabolites in serum and urine by reversed-phase ion-pair chromatography. J Chromatogr 199:345–354. https://doi.org/10.1016/S0021-9673(01)91386-X
Agostini JF, Toé HCZD, Vieira KM, Baldin SL, Costa NLF, Cruz CU, Longo L, Machado MM, da Silveira TR, Schuck PF, Rico EP (2018) Cholinergic system and oxidative stress changes in the brain of a zebrafish model chronically exposed to ethanol. Neurotox Res 33:749–758. https://doi.org/10.1007/s12640-017-9816-8
Dal Santo G, Grotto A, Boligon AA, Da Costa B, Rambo CL, Fantini EE, Lazzarotto LMV, Bertoncello KT, JúniorOT GSC, Siebel AM, Rosemberg DB, Magro JD, Conterato GMM, Zanatta L (2018) Protective effect of Uncaria tomentosa extract against oxidative stress and genotoxicity induced by glyphosate-Roundup® using zebrafish (Danio rerio) as a model. Environ Sci Pollut Res Int 25:11703–11715. https://doi.org/10.1007/s11356-018-1350-6
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. https://doi.org/10.1016/0076-6879(90)86134-h
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2', 7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231. https://doi.org/10.1021/tx00026a012
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145. https://doi.org/10.1016/s0304-3940(01)01636-6
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Bannister JV, Calabrese L (1987) Assays for superoxide dismutase. Methods Biochem Anal 32:279–312. https://doi.org/10.1002/9780470110539.ch5
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356. https://doi.org/10.1016/0003-2697(77)90043-4
De Witte P (2004) Imbalance between neuroexcitatory and neuroinhibitory amino acids causes craving for ethanol. Addict Behav 29(7):1325–1339
Quertemont E, Tambour S, Tirelli E (2005) The role of acetaldehyde in the neurobehavioral effects of ethanol: a comprehensive review of animal studies. Prog Neurobiol 75:247–274. https://doi.org/10.1016/j.addbeh.2004.06.020
Gonzalez RA, Jaworski JN (1997) Alcohol and glutamate. Alcohol Health Res World 21(2):120–127
Rao PSS, Sari Y (2012) Glutamate transporter 1: target for the treatment of alcohol dependence. Curr Med Chem 19:5148–5156. https://doi.org/10.2174/092986712803530511
Bantel C, Childers SR, Eisenach JC (2002) Role of adenosine receptors in spinal G-protein activation after peripheral nerve injury. Anesthesiology. 96:1443–1449. https://doi.org/10.1097/00000542-200206000-00025
Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP (2001) International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev 2001(53):107–118
Asatryan L, Nam HW, Lee MR, Thakkar MM, Saeed Dar M, Davies DL, Choi DS (2011) Implication of the purinergic system in alcohol use disorders. Alcohol Clin Exp Res 35:584–594. https://doi.org/10.1111/j.1530-0277.2010.01379.x
Rico EP, Rosemberg DB, Seibt KJ, Capiotti KM, Da Silva RS, Bonan CD (2011) Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. Neurotoxicol Teratol 33:608–617. https://doi.org/10.1016/j.ntt.2011.07.007
Zimmermann H (2021) Ectonucleoside triphosphate diphosphohydrolases and ecto-5'-nucleotidase in purinergic signaling: how the field developed and where we are now. Purinergic Signal 17:117–125. https://doi.org/10.1007/s11302-020-09755-6
Barbosa KBF, Costa NMB, De Cássia Gonçalves Alfenas R, De Paula SO, Minim VPR, Bressan J (2010) Estresse oxidativo: Conceito, implicações e fatores modulatórios. Rev Nutr 23:629–643. https://doi.org/10.1590/S1415-52732010000400013
Chandrasekhar Y, Phani Kumar G, Ramya EM, Anilakumar KR (2018) Gallic acid protects 6-OHDA induced neurotoxicity by attenuating oxidative stress in human dopaminergic cell line. Neurochem Res 43:1150–1160. https://doi.org/10.1007/s11064-018-2530-y
Kosuru RY, Roy A, Das SK, Bera S (2018) Gallic acid and gallates in human health and disease: do mitochondria hold the key to success? Mol Nutr Food Res 62. https://doi.org/10.1002/mnfr.201700699
Lovinger DM, White G, Weight FF (1989) Ethanol inhibits NMDAactivated ion current in hippocampal neurons. Science 243(4899):1721–1724
Deitrich RA (2004) Acetaldehyde: deja vu du jour. J Stud Alcohol 65:557–572
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine. Free Radic Biol Med. https://doi.org/10.1093/acprof:oso/9780198717478.001.0001
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) research Grant (Universal 429302/2018-5), Fundação de Amparo à Pesquisa do Estado de Santa Catarina (FAPESC - Universal 12/2020), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES AUXPE PROEX N. 23038.020053/2018-52).
Author information
Authors and Affiliations
Contributions
SLB: investigation, validation, data curation, writing - original draft. KPP: conceptualization, concept and design. ACSF: investigation, validation, data curation, writing - original draft. HTB: formal analysis investigation, writing - review and editing. RS: data analysis and interpretation, writing - review and editing. BCP: investigation, validation, data curation. SDP: investigation, validation, data curation. ERD: formal analysis investigation, writing - review & editing. RAM: formal analysis investigation, writing - review and editing. AGW: formal analysis investigation, writing - review and editing. EPR: funding acquisition, project administration, supervision, writing — review and editing, resources. The paper was read, revised, and approved by all the authors.
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The study protocol was approved by the Ethics Committee of University of Southern Santa Catarina (UNESC), Criciúma, Brazil, number 030/2019-1.
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Gallic acid reduces oxidative stress induced by acute ethanol in the zebrafish brain.
• Gallic acid prevents disruption of NTPDase activity promoted by ethanol.
• Ethanol alters the degradation of extracellular nucleotides in the zebrafish brain.
Rights and permissions
About this article
Cite this article
Baldin, S.L., de Pieri Pickler, K., de Farias, A.C.S. et al. Gallic acid modulates purine metabolism and oxidative stress induced by ethanol exposure in zebrafish brain. Purinergic Signalling 18, 307–315 (2022). https://doi.org/10.1007/s11302-022-09869-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-022-09869-z