Abstract
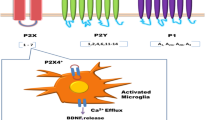
The comorbid mechanism of depression and chronic pain has been a research hotspot in recent years. Until now, the role of purinergic signals in the comorbid mechanism of depression and chronic pain has not been fully understood. This review mainly summarizes the research results published in PubMed during the past 5 years and concludes that purinergic signaling is a potential therapeutic target for comorbid depression and chronic pain, and the purinergic receptors A1, A2A, P2X3, P2X4, and P2X7and P2Y6, P2Y1, and P2Y12 may be important factors. The main potential pathways are as follows: A1 receptor-related G protein-dependent activation of introverted K+ channels (GIRKs), A2A receptor-related effects on the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and MAPK/nuclear factor-κB (NF-κB) pathways, P2X3 receptor-related effects on dorsal root ganglia (DRG) excitability, P2X4 receptor-related effects on proinflammatory cytokines and inflammasome activation, P2X7 receptor-related effects on ion channels, the NLRP3 inflammasome and brain-derived neurotrophic factor (BDNF), and P2Y receptor-related effects on the phospholipase C (PLC)/inositol triphosphate (IP3)/Ca2+ signaling pathway. We hope that the conclusions of this review will provide key ideas for future research on the role of purinergic signaling in the comorbid mechanism of depression and chronic pain.


Similar content being viewed by others
Data availability
Not applicable.
References
Suleman R, Tucker BV, Dursun SM, Demas ML (2021) The neurostimulation of the brain in depression trial: protocol for a randomized controlled trial of transcranial direct current stimulation in treatment-resistant depression. JMIR Res Protoc 10(3):e22805. https://doi.org/10.2196/22805
Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, Yu Y, Kou C, Xu X, Lu J, Wang Z, He S, Xu Y, He Y, Li T, Guo W, Tian H, Xu G, Xu X, Ma Y, Wang L, Wang L, Yan Y, Wang B, Xiao S, Zhou L, Li L, Tan L, Zhang T, Ma C, Li Q, Ding H, Geng H, Jia F, Shi J, Wang S, Zhang N, Du X, Du X, Wu Y (2019) Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry 6(3):211–224. https://doi.org/10.1016/S2215-0366(18)30511-X
Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ (2019) Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 160(1):19–27. https://doi.org/10.1097/j.pain.0000000000001384
Wohleb ES (2016) Neuron-microglia interactions in mental health disorders: “For Better, and For Worse.” Front Immunol 7:544. https://doi.org/10.3389/fimmu.2016.00544
Lee J, Bae JY, Lee CJ, Bae YC (2018) Electrophysiological evidence for functional astrocytic P2X3 receptors in the mouse trigeminal caudal nucleus. Exp Neurobiol 27(2):88–93. https://doi.org/10.5607/en.2018.27.2.88
Mills SEE, Nicolson KP, Smith BH (2019) Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth 123(2):e273–e283. https://doi.org/10.1016/j.bja.2019.03.023
Abbracchio MP (2021) The history of the Purine Club: a tribute to Prof. Geoffrey Burnstock. Purinergic Signal 17(1):127–134. https://doi.org/10.1007/s11302-020-09749-4
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24(3):509–581
Burnstock G (2017) Purinergic signalling: therapeutic developments. Front Pharmacol 8:661. https://doi.org/10.3389/fphar.2017.00661
Alves M, Smith J, Engel T (2019) Differential expression of the metabotropic P2Y receptor family in the cortex following status epilepticus and neuroprotection via P2Y1 antagonism in mice. Front Pharmacol 10:1558. https://doi.org/10.3389/fphar.2019.01558
Ortiz R, Ulrich H, Zarate CA Jr, Machado-Vieira R (2015) Purinergic system dysfunction in mood disorders: a key target for developing improved therapeutics. Prog Neuropsychopharmacol Biol Psychiatry 57:117–31. https://doi.org/10.1016/j.pnpbp.2014.10.016
Zhang, W.J., H.L. Luo, and Z.M. Zhu (2020) The role of P2X4 receptors in chronic pain: a potential pharmacological target. Biomed Pharmacother 129: 110447. https://doi.org/10.1016/j.biopha.2020.110447
Trang, M., G. Schmalzing, C.E. Muller, and F. Markwardt (2020) Dissection of P2X4 and P2X7 receptor current components in BV-2 microglia. Int J Mol Sci 21 (22). https://doi.org/10.3390/ijms21228489
Kyrargyri V, Madry C, Rifat A, Arancibia-Carcamo IL, Jones SP, Chan VTT, Xu Y, Robaye B, Attwell D (2020) P2Y13 receptors regulate microglial morphology, surveillance, and resting levels of interleukin 1beta release. Glia 68(2):328–344. https://doi.org/10.1002/glia.23719
Lalo U, Bogdanov A, Pankratov Y (2019) Age- and experience-related plasticity of ATP-mediated signaling in the neocortex. Front Cell Neurosci 13:242. https://doi.org/10.3389/fncel.2019.00242
Lee, D.S. and J.E. Kim (2020) P2 x 7 Receptor inhibits astroglial autophagy via regulating FAK- and PHLPP1/2-mediated AKT-S473 phosphorylation following kainic acid-induced seizures. Int J Mol Sci 21 (18). https://doi.org/10.3390/ijms21186476
Zhou F, Liu X, Gao L, Zhou X, Cao Q, Niu L, Wang J, Zuo D, Li X, Yang Y, Hu M, Yu Y, Tang R, Lee BH, Choi BW, Wang Y, Izumiya Y, Xue M, Zheng K, Gao D (2019) HIV-1 Tat enhances purinergic P2Y4 receptor signaling to mediate inflammatory cytokine production and neuronal damage via PI3K/Akt and ERK MAPK pathways. J Neuroinflammation 16(1):71. https://doi.org/10.1186/s12974-019-1466-8
Kinoshita M, Hirayama Y, Fujishita K, Shibata K, Shinozaki Y, Shigetomi E, Takeda A, Le HPN, Hayashi H, Hiasa M, Moriyama Y, Ikenaka K, Tanaka KF, Koizumi S (2018) Anti-depressant fluoxetine reveals its therapeutic effect via astrocytes. EBioMedicine 32:72–83. https://doi.org/10.1016/j.ebiom.2018.05.036
Quintas C, Vale N, Goncalves J, Queiroz G (2018) Microglia P2Y13 receptors prevent astrocyte proliferation mediated by P2Y1 receptors. Front Pharmacol 9:418. https://doi.org/10.3389/fphar.2018.00418
Campos ACP, Antunes GF, Matsumoto M, Pagano RL, Martinez RCR (2020) Neuroinflammation, pain and depression: an overview of the main findings. Front Psychol 11:1825. https://doi.org/10.3389/fpsyg.2020.01825
Yirmiya R, Rimmerman N, Reshef R (2015) Depression as a microglial disease. Trends Neurosci 38(10):637–658. https://doi.org/10.1016/j.tins.2015.08.001
Ji RR, Xu ZZ, Gao YJ (2014) Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 13(7):533–48. https://doi.org/10.1038/nrd4334
Fritz BM, Yin F, Atwood BK (2021) Input-selective adenosine A1 receptor-mediated synaptic depression of excitatory transmission in dorsal striatum. Sci Rep 11(1):6345. https://doi.org/10.1038/s41598-021-85513-x
Borea PA, Gessi S, Merighi S, Varani K (2016) Adenosine as a multi-signalling guardian angel in human diseases: when, where and how does it exert its protective effects? Trends Pharmacol Sci 37(6):419–434. https://doi.org/10.1016/j.tips.2016.02.006
Serchov T, Clement HW, Schwarz MK, Iasevoli F, Tosh DK, Idzko M, Jacobson KA, de Bartolomeis A, Normann C, Biber K, van Calker D (2015) Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of Homer1a. Neuron 87(3):549–62. https://doi.org/10.1016/j.neuron.2015.07.010
Akiyama Y, Luo Y, Hanno PM, Maeda D, Homma Y (2020) Interstitial cystitis/bladder pain syndrome: the evolving landscape, animal models and future perspectives. Int J Urol 27(6):491–503. https://doi.org/10.1111/iju.14229
Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor J, Wallach I, Nairn AC, Surmeier DJ, Greengard P (2008) FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat Neurosci 11(12):1402–9. https://doi.org/10.1038/nn.2216
Ko IG, Jin JJ, Hwang L, Kim SH, Kim CJ, Won KY, Na YG, Kim KH, Kim SJ (2021) Adenosine A2A receptor agonist polydeoxyribonucleotide alleviates interstitial cystitis-induced voiding dysfunction by suppressing inflammation and apoptosis in rats. J Inflamm Res 14:367–378. https://doi.org/10.2147/JIR.S287346
Hamilton LJ, Walker M, Pattabiraman M, Zhong HA, Luedtke B, Chandra S (2021) Novel curcumin analog (cis-trans curcumin) as ligand to adenosine receptors A2A and A2B: potential for therapeutics. Pharmacol Res 165:105410. https://doi.org/10.1016/j.phrs.2020.105410
Guida F, Luongo L, Marmo F, Romano R, Iannotta M, Napolitano F, Belardo C, Marabese I, D’Aniello A, De Gregorio D, Rossi F, Piscitelli F, Lattanzi R, de Bartolomeis A, Usiello A, Di Marzo V, de Novellis V, Maione S (2015) Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol Brain 8:47. https://doi.org/10.1186/s13041-015-0139-5
Ohtori, S., K. Takahashi, H. Moriya, and R.R. Myers (2004) TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine (Phila Pa 1976) 29 (10): 1082–8. https://doi.org/10.1097/00007632-200405150-00006
Koizumi M, Asano S, Furukawa A, Hayashi Y, Hitomi S, Shibuta I, Hayashi K, Kato F, Iwata K, Shinoda M (2021) P2X3 receptor upregulation in trigeminal ganglion neurons through TNFalpha production in macrophages contributes to trigeminal neuropathic pain in rats. J Headache Pain 22(1):31. https://doi.org/10.1186/s10194-021-01244-4
Guan S, Shen Y, Ge H, Xiong W, He L, Liu L, Yin C, Wei X, Gao Y (2019) Dihydromyricetin alleviates diabetic neuropathic pain and depression comorbidity symptoms by inhibiting P2X7 receptor. Front Psychiatry 10:770. https://doi.org/10.3389/fpsyt.2019.00770
Zhang X, Chen Y, Wang C, Huang LY (2007) Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A 104(23):9864–9. https://doi.org/10.1073/pnas.0611048104
Ford AP (2012) In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal 8(Suppl 1):3–26. https://doi.org/10.1007/s11302-011-9271-6
Morice, A.H., M.M. Kitt, A.P. Ford, A.M. Tershakovec, W.C. Wu, K. Brindle, R. Thompson, S. Thackray-Nocera, and C. Wright (2019) The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J 54 (1). https://doi.org/10.1183/13993003.00439-2019
Wang Y, Mackes J, Chan S, Haughey NJ, Guo Z, Ouyang X, Furukawa K, Ingram DK, Mattson MP (2006) Impaired long-term depression in P2X3 deficient mice is not associated with a spatial learning deficit. J Neurochem 99(5):1425–34. https://doi.org/10.1111/j.1471-4159.2006.04198.x
Wang M, Cai X, Wang Y, Li S, Wang N, Sun R, Xing J, Liang S, Liu S (2020) Astragalin alleviates neuropathic pain by suppressing P2X4-mediated signaling in the dorsal root ganglia of rats. Front Neurosci 14:570831. https://doi.org/10.3389/fnins.2020.570831
Li L, Zou Y, Liu B, Yang R, Yang J, Sun M, Li Z, Xu X, Li G, Liu S, Greffrath W, Treede RD, Li G, Liang S (2020) Contribution of the P2X4 receptor in rat hippocampus to the comorbidity of chronic pain and depression. ACS Chem Neurosci 11(24):4387–4397. https://doi.org/10.1021/acschemneuro.0c00623
Yu Y, Feng L, Li J, Lan X, L. A, X. Lv, M. Zhang, and L. Chen, (2017) The alteration of autophagy and apoptosis in the hippocampus of rats with natural aging-dependent cognitive deficits. Behav Brain Res 334:155–162. https://doi.org/10.1016/j.bbr.2017.07.003
Xie L, Gu Z, Liu H, Jia B, Wang Y, Cao M, Song R, Zhang Z, Bian Y (2020) The anti-depressive effects of hesperidin and the relative mechanisms based on the NLRP3 inflammatory signaling pathway. Front Pharmacol 11:1251. https://doi.org/10.3389/fphar.2020.01251
Wang H, Guo W, Liu H, Zeng R, Lu M, Chen Z, Xiao Q (2013) Inhibition of inflammatory mediator release from microglia can treat ischemic/hypoxic brain injury. Neural Regen Res 8(13):1157–68. https://doi.org/10.3969/j.issn.1673-5374.2013.13.001
Shen Y, Guan S, Ge H, Xiong W, He L, Liu L, Yin C, Liu H, Li G, Xu C, Xu H, Liu S, Li G, Liang S, Gao Y (2018) Effects of palmatine on rats with comorbidity of diabetic neuropathic pain and depression. Brain Res Bull 139:56–66. https://doi.org/10.1016/j.brainresbull.2018.02.005
Chu YX, Zhang Y, Zhang YQ, Zhao ZQ (2010) Involvement of microglial P2X7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain Behav Immun 24(7):1176–89. https://doi.org/10.1016/j.bbi.2010.06.001
Yue N, Huang H, Zhu X, Han Q, Wang Y, Li B, Liu Q, Wu G, Zhang Y, Yu J (2017) Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation 14(1):102. https://doi.org/10.1186/s12974-017-0865-y
Mehta N, Kaur M, Singh M, Chand S, Vyas B, Silakari P, Bahia MS, Silakari O (2014) Purinergic receptor P2X(7): a novel target for anti-inflammatory therapy. Bioorg Med Chem 22(1):54–88. https://doi.org/10.1016/j.bmc.2013.10.054
Ribeiro DE, Muller HK, Elfving B, Eskelund A, Joca SR, Wegener G (2019) Antidepressant-like effect induced by P2X7 receptor blockade in FSL rats is associated with BDNF signalling activation. J Psychopharmacol 33(11):1436–1446. https://doi.org/10.1177/0269881119872173
Kuan YH, Shih HC, Shyu BC (2018) Involvement of P2X7 receptors and BDNF in the pathogenesis of central poststroke pain. Adv Exp Med Biol 1099:211–227. https://doi.org/10.1007/978-981-13-1756-9_18
Glaser T, Andrejew R, Oliveira-Giacomelli A, Ribeiro DE, Bonfim Marques L, Ye Q, Ren WJ, Semyanov A, Illes P, Tang Y, Ulrich H (2020) Purinergic receptors in basal ganglia diseases: shared molecular mechanisms between Huntington’s and Parkinson’s disease. Neurosci Bull 36(11):1299–1314. https://doi.org/10.1007/s12264-020-00582-8
Iqubal A, Ahmed M, Iqubal MK, Pottoo FH, Haque SE (2020) Polyphenols as potential therapeutics for pain and inflammation in spinal cord injury. Curr Mol Pharmacol. https://doi.org/10.2174/1874467213666201223111743
Magni G, Ceruti S (2013) P2Y purinergic receptors: new targets for analgesic and antimigraine drugs. Biochem Pharmacol 85(4):466–77. https://doi.org/10.1016/j.bcp.2012.10.027
Malin SA, Molliver DC (2010) Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain 6:21. https://doi.org/10.1186/1744-8069-6-21
Rajani V, Zhang Y, Jalubula V, Rancic V, SheikhBahaei S, Zwicker JD, Pagliardini S, Dickson CT, Ballanyi K, Kasparov S, Gourine AV, Funk GD (2018) Release of ATP by pre-Botzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca(2+) -dependent P2Y1 receptor mechanism. J Physiol 596(15):3245–3269. https://doi.org/10.1113/JP274727
Chen Y, Zhang X, Wang C, Li G, Gu Y, Huang LY (2008) Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci U S A 105(43):16773–8. https://doi.org/10.1073/pnas.0801793105
Xu X, Liu B, Yang J, Zou Y, Sun M, Li Z, Li L, Yang R, Zou L, Li G, Liu S, Li G, Liang S (2020) Glucokinase in stellate ganglia cooperates with P2X3 receptor to develop cardiac sympathetic neuropathy in type 2 diabetes rats. Brain Res Bull 165:290–297. https://doi.org/10.1016/j.brainresbull.2020.10.004
Mikuzuki L, Saito H, Katagiri A, Okada S, Sugawara S, Kubo A, Ohara K, Lee J, Toyofuku A, Iwata K (2017) Phenotypic change in trigeminal ganglion neurons associated with satellite cell activation via extracellular signal-regulated kinase phosphorylation is involved in lingual neuropathic pain. Eur J Neurosci 46(6):2190–2202. https://doi.org/10.1111/ejn.13667
Amadio S, Parisi C, Montilli C, Carrubba AS, Apolloni S, Volonte C (2014) P2Y(12) receptor on the verge of a neuroinflammatory breakdown. Mediators Inflamm 2014:975849. https://doi.org/10.1155/2014/975849
Inoue K (2017) Purinergic signaling in microglia in the pathogenesis of neuropathic pain. Proc Jpn Acad Ser B Phys Biol Sci 93(4):174–182. https://doi.org/10.2183/pjab.93.011
Walker AK, Kavelaars A, Heijnen CJ, Dantzer R (2014) Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev 66(1):80–101. https://doi.org/10.1124/pr.113.008144
Popiolek-Barczyk K, Mika J (2016) Targeting the microglial signaling pathways: new insights in the modulation of neuropathic pain. Curr Med Chem 23(26):2908–2928. https://doi.org/10.2174/0929867323666160607120124
Long T, He W, Pan Q, Zhang S, Zhang D, Qin G, Chen L, Zhou J (2020) Microglia P2X4R-BDNF signalling contributes to central sensitization in a recurrent nitroglycerin-induced chronic migraine model. J Headache Pain 21(1):4. https://doi.org/10.1186/s10194-019-1070-4
Hu X, Dong Y, Jin X, Zhang C, Zhang T, Zhao J, Shi J, Li J (2017) The novel and potent anti-depressive action of triptolide and its influences on hippocampal neuroinflammation in a rat model of depression comorbidity of chronic pain. Brain Behav Immun 64:180–194. https://doi.org/10.1016/j.bbi.2017.03.005
Zhu C, Xu Q, Mao Z, Lin N (2018) The Chinese medicine Wu-Tou decoction relieves neuropathic pain by inhibiting hippocampal microglia activation. Sci Rep 8(1):12292. https://doi.org/10.1038/s41598-018-30006-7
Huang Z, Xie N, Illes P, Di Virgilio F, Ulrich H, Semyanov A, Verkhratsky A, Sperlagh B, Yu SG, Huang C, Tang Y (2021) From purines to purinergic signalling: molecular functions and human diseases. Signal Transduct Target Ther 6(1):162. https://doi.org/10.1038/s41392-021-00553-z
Zarrinmayeh H, Territo PR (2020) Purinergic receptors of the central nervous system: biology, PET ligands, and their applications. Mol Imaging 19:1536012120927609. https://doi.org/10.1177/1536012120927609
Illes P, Verkhratsky A, Tang Y (2019) Pathological ATPergic signaling in major depression and bipolar disorder. Front Mol Neurosci 12:331. https://doi.org/10.3389/fnmol.2019.00331
Ribeiro, D.E., A.L. Roncalho, T. Glaser, H. Ulrich, G. Wegener, and S. Joca (2019) P2X7 receptor signaling in stress and depression. Int J Mol Sci 20 (11). https://doi.org/10.3390/ijms20112778
Funding
This work was supported by grants (Nos. 81861138042, 81870574, 81570735, and 31560276) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zou, Y., Yang, R., Li, L. et al. Purinergic signaling: a potential therapeutic target for depression and chronic pain. Purinergic Signalling 19, 163–172 (2023). https://doi.org/10.1007/s11302-021-09801-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-021-09801-x