Abstract
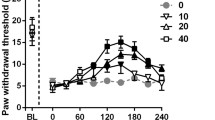
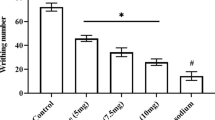
This study aimed to evaluate the effect of a single administration of IB-MECA, an A3 adenosine receptor agonist, upon the nociceptive response and central biomarkers of rats submitted to chronic pain models. A total of 136 adult male Wistar rats were divided into two protocols: (1) chronic inflammatory pain (CIP) using complete Freund’s adjuvant and (2) neuropathic pain (NP) by chronic constriction injury of the sciatic nerve. Thermal and mechanical hyperalgesia was measured using von Frey (VF), Randal-Selitto (RS), and hot plate (HP) tests. Rats were treated with a single dose of IB-MECA (0.5 μmol/kg i.p.), a vehicle (dimethyl sulfoxide—DMSO), or positive control (morphine, 5 mg/kg i.p.). Interleukin 1β (IL-1β), brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF) levels were measured in the brainstem and spinal cord using enzyme-linked immunosorbent assay (ELISA). The establishment of the chronic pain (CIP or NP) model was observed 14 days after induction by a decreased nociceptive threshold in all three tests (GEE, P < 0.05). The antinociceptive effect of a single dose of IB-MECA was observed in both chronic pain models, but this was more effective in NP model. There was an increase in IL-1β levels promoted by CIP. NP model promoted increase in the brainstem BDNF levels, which was reversed by IB-MECA













Similar content being viewed by others
References
Cohen SP, Mao J (2014) Neuropathic pain : mechanisms and their clinical implications. Br J Med 348:1–12
Zylka MJ (2011) Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med 17:188–196
Burnstock G (2015) An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology 104:4–17
Burnstock G (2016) Purinergic mechanisms and pain. Adv Pharmacol 75:91–137
Sawynok J (2016) Adenosine receptor targets for pain. Neuroscience 338:1–18
Jacobson KA, Gao Z-G (2006) Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5:247–264
Borea PA, Varani K, Vincenzi F et al (2014) The A3 adenosine receptor: history and perspectives. Pharmacol Rev 67:74–102
Cohen S, Barer F, Bar-Yehuda S, IJzerman AP, Jacobson KA, Fishman P (2014) A3 adenosine receptor allosteric modulator induces an anti-inflammatory effect: in vivo studies and molecular mechanism of action. Mediat Inflamm 2014:1–8
Yoon MH, Bae HB, Choi J II (2005) Antinociception of intrathecal adenosine receptor subtype agonists in rat formalin test. Anesth Analg 101:1417–1421
Chen Z, Janes K, Chen C, Doyle T, Bryant L, Tosh DK, Jacobson KA, Salvemini D (2012) Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J 26:1855–1865
Janes K, Esposito E, Doyle T, Cuzzocrea S, Tosh DK, Jacobson KA, Salvemini D (2014) A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain 155:2560–2567
Ellis A, Bennett DLH (2013) Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 111:26–37
Clark AK, Old E, Malcangio M (2013) Neuropathic pain and cytokines: current perspectives. J Pain Res 6:803–814
Huh Y, Ji R-R, Chen G (2017) Neuroinflammation, bone marrow stem cells, and chronic pain. Front Immunol 8
Apkarian AV, Lavarello S, Randolf A et al (2006) Expression of IL-1beta in supraspinal brain regions in rats with neuropathic pain. Neurosci Lett 407:176–181
del Rey A, Yau H-J, Randolf A, Centeno MV, Wildmann J, Martina M, Besedovsky HO, Apkarian AV (2011) Chronic neuropathic pain-like behavior correlates with IL-1β expression and disrupts cytokine interactions in the hippocampus. Pain 152:2827–2835
del Rey A, Apkarian AV, Martina M, Besedovsky HO (2012) Chronic neuropathic pain-like behavior and brain-borne IL-1β. Ann N Y Acad Sci 1262:101–107
Khan N, Smith MT (2015) Neurotrophins and neuropathic pain: role in pathobiology. Molecules 20:10657–10688
Pezet S, McMahon SB (2006) Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29:507–538
Torres I, Buffon A, Silveira PP et al (2002) Effect of chronic and acute stress on ectonucleotidase activities in spinal cord. Physiol Behav 75:1–5
Medeiros LF, Rozisky JR, de Souza A, Hidalgo MP, Netto CA, Caumo W, Battastini AMO, Torres ILS (2011) Lifetime behavioural changes after exposure to anaesthetics in infant rats. Behav Brain Res 218:51–56
Torres I, Cucco SNS, Bassani M et al (2003) Long-lasting delayed hyperalgesia after chronic restraint stress in rats—effect of morphine administration. Neurosci Res 45:277–283
Kilkenny C, Browne WJ, Cuthill IC et al (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:1–6
Laste G, Macedo IC, Rozisky JR et al (2012) Melatonin administration reduces inflammatory pain in rats. J Pain Res 5:359–362
Guzman-silva MA, Pollastri CE, Augusto J et al (2008) Tramadol minimizes potential pain during post-oophorectomy in Wistar rats. AATEX 14:91–92
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Cioato SG, Medeiros LF, Marques Filho PR, Vercelino R, de Souza A, Scarabelot VL, de Oliveira C, Adachi LNS, Fregni F, Caumo W, Torres ILS (2016) Long-lasting effect of transcranial direct current stimulation in the reversal of hyperalgesia and cytokine alterations induced by the neuropathic pain model. Brain Stimul 9:209–217
Rozisky JR, Dantas G, Adachi LS, Alves VS, Ferreira MBC, Sarkis JJF, Torres ILDS (2008) Long-term effect of morphine administration in young rats on the analgesic opioid response in adult life. Int J Dev Neurosci 26:561–565
Vivancos GG, Verri WA, Cunha TM et al (2004) An electronic pressure-meter nociception paw test for rats. Brazilian J Med Biol Res 37:39139–39139
Randall LO, Selitto JJ (1957) A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 111:409–419
Woolfe G, Macdonald A (1944) The evaluation of the analgesic action of pethidine hydrochloride. J Pharmacol Exp Ther 80:300–307
Ossipov MH, Kovelowski CJ, Nichols ML, Hruby VJ, Porreca F (1995) Characterization of supraspinal antinociceptive actions of opioid delta agonists in the rat. Pain 62:287–293
Caggiula AR, Epstein LH, Perkins KA, Saylor S (1995) Different methods of assessing nicotine-induced antinociception may engage different neural mechanisms. Psychopharmacology 122:301–306
Rubinstein M, Mogil JS, Japón M et al (1996) Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci U S A 93:3995–4000
Netto CA, Siegfried B, Izquierdo I (1987) Analgesia induced by exposure to a novel environment in rats: effect of concurrent and post-training stressful stimulation. Behav Neural Biol 48:304–309
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ballinger GA (2004) Using generalized estimating equations for longitudinal data analysis. Organ Res Methods 7:127–150. https://doi.org/10.1177/1094428104263672
Sawynok J, Liu XJ (2003) Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol 69:313–340
Sawynok J, Zarrindast MR, Reid AR, Doak GJ (1997) Adenosine A3 receptor activation produces nociceptive behaviour and edema by release of histamine and 5-hydroxytryptamine. Eur J Pharmacol 333:1–7
Little JW, Ford A, Symons-Liguori AM, Chen Z, Janes K, Doyle T, Xie J, Luongo L, Tosh DK, Maione S, Bannister K, Dickenson AH, Vanderah TW, Porreca F, Jacobson KA, Salvemini D (2015) Endogenous adenosine A3 receptor activation selectively alleviates persistent pain states. Brain 138:28–35
Janes K, Symons-Liguori AM, Jacobson KA, Salvemini D (2016) Identification of A3 adenosine receptor agonists as novel non-narcotic analgesics. Br J Pharmacol 173:1253–1267
Sachdeva S, Gupta M (2013) Adenosine and its receptors as therapeutic targets: an overview. Saudi Pharm J 21:245–253
Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA (2008) The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther 117:123–140
Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K (2018) Pharmacology of adenosine receptors: the state of the art. Physiol Rev 98:1591–1625
Terayama R, Tabata M, Maruhama K, Iida S (2018) A3 adenosine receptor agonist attenuates neuropathic pain by suppressing activation of microglia and convergence of nociceptive inputs in the spinal dorsal horn. Exp Brain Res 236:3203–3213
Janes K, Wahlman C, Little JW et al (2015) Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav Immun 44:91–99
Ren T, Tian T, Feng X et al (2015) An adenosine A3 receptor agonist inhibits DSS-induced colitis in mice through modulation of the NF-κB signaling pathway. Sci Rep 5:9047
Tian Hua R, Min Min L, Xiao Meng A et al (2019) Activation of adenosine A3 receptor inhibits inflammatory cytokine production in colonic mucosa of patients with ulcerative colitis by down-regulating the nuclear factor-kappa B signaling. J Dig Dis 21:38–45
Ren T, Qiu Y, Wu W et al (2014) Activation of adenosine A3 receptor alleviates TNF- alpha -induced inflammation through inhibition of the NF-kB signaling pathway in human colonic epithelial cells. Mediat Inflamm 2014:1–11
Jacobson KA, Merighi S, Varani K et al (2018) A3 adenosine receptors as modulators of inflammation: from medicinal chemistry to therapy. Med Res Rev 38:1031–1072
Fishman P, Bar-Yehuda S (2003) Pharmacology and therapeutic applications of A3 receptor subtype. Curr Top Med Chem 3:463–469
Fishman P, Bar-Yehuda S, Madi L et al (2006) The PI3K-NF-κB signal transduction pathway is involved in mediating the anti-inflammatory effect of IB-MECA in adjuvant-induced arthritis. Arthritis Res Ther 8:1–9
Ohsawa K, Sanagi T, Nakamura Y, Suzuki E, Inoue K, Kohsaka S (2012) Adenosine A3 receptor is involved in ADP-induced microglial process extension and migration. J Neurochem 121:217–227
Milligan ED, Watkins LR (2009) Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 10:23–36
Merighi A, Salio C, Ghirri A et al (2008) BDNF as a pain modulator. Prog Neurobiol 85:297–317
Coull JAM, Beggs S, Boudreau D et al (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438:1017–1021
Haskó G, Linden J, Cronstein B, Pacher P (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7:759–770
Haskó G, Szabó C, Németh ZH et al (1996) Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol 157:4634–4640
Woolf CJ, Allchorne A, Poole S (1997) Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor a 1. Br J Pharmacol 121:417–424
Ma QP, Woolf CJ (1996) Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain 67:97–106
Laste G, de Souza ICC, dos Santos VS et al (2014) Histopathological changes in three variations of Wistar rat adjuvant-induced arthritis model. Int J Pharm Res Sch 3:780–90
Raghavendra V, Tanga FY, DeLeo JA (2004) Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci 20:467–473
Holló K, Ducza L, Hegyi Z et al (2017) Interleukin-1 receptor type 1 is overexpressed in neurons but not in glial cells within the rat superficial spinal dorsal horn in complete Freund adjuvant-induced inflammatory pain. J Neuroinflammation 14:1–18
Gunn A, Bobeck EN, Weber C, Morgan MM (2012) The influence of non-nociceptive factors on hot plate latency in rats. J Pain 12:222–227
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Stefania Giotti Cioato declares that she has no conflict of interest. Liciane Fernandes Medeiros declares that she has no conflict of interest. Bettega Costa Lopes declares that he has no conflict of interest. Andressa de Souza declares that she has no conflict of interest. Helouise Richardt Medeiros declares that she has no conflict of interest. José Antônio Fagundes Assumpção declares that he has no conflict of interest. Wolnei Caumo declares that he has no conflict of interest. Rafael Roesler declares that he has no conflict of interest. Iraci L. S. Torres declares that she has no conflict of interest.
Ethical approval
This study was approved by the Institutional Animal Care and Use Committee (GPPG-HCPA protocol no. 150530 and no.2018-0377).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cioato, S.G., Medeiros, L.F., Lopes, B.C. et al. Antinociceptive and neurochemical effects of a single dose of IB-MECA in chronic pain rat models. Purinergic Signalling 16, 573–584 (2020). https://doi.org/10.1007/s11302-020-09751-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-020-09751-w