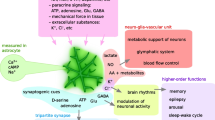
Abstract
Caffeine, a stimulant largely consumed around the world, is a non-selective adenosine receptor antagonist, and therefore caffeine actions at synapses usually, but not always, mirror those of adenosine. Importantly, different adenosine receptors with opposing regulatory actions co-exist at synapses. Through both inhibitory and excitatory high-affinity receptors (A1R and A2R, respectively), adenosine affects NMDA receptor (NMDAR) function at the hippocampus, but surprisingly, there is a lack of knowledge on the effects of caffeine upon this ionotropic glutamatergic receptor deeply involved in both positive (plasticity) and negative (excitotoxicity) synaptic actions. We thus aimed to elucidate the effects of caffeine upon NMDAR-mediated excitatory post-synaptic currents (NMDAR-EPSCs), and its implications upon neuronal Ca2+ homeostasis. We found that caffeine (30–200 μM) facilitates NMDAR-EPSCs on pyramidal CA1 neurons from Balbc/ByJ male mice, an action mimicked, as well as occluded, by 1,3-dipropyl-cyclopentylxantine (DPCPX, 50 nM), thus likely mediated by blockade of inhibitory A1Rs. This action of caffeine cannot be attributed to a pre-synaptic facilitation of transmission because caffeine even increased paired-pulse facilitation of NMDA-EPSCs, indicative of an inhibition of neurotransmitter release. Adenosine A2ARs are involved in this likely pre-synaptic action since the effect of caffeine was mimicked by the A2AR antagonist, SCH58261 (50 nM). Furthermore, caffeine increased the frequency of Ca2+ transients in neuronal cell culture, an action mimicked by the A1R antagonist, DPCPX, and prevented by NMDAR blockade with AP5 (50 μM). Altogether, these results show for the first time an influence of caffeine on NMDA receptor activity at the hippocampus, with impact in neuronal Ca2+ homeostasis.






Similar content being viewed by others
Data availability
Not applicable
Abbreviations
- A1R:
-
Adenosine A1 receptor
- A2AR:
-
Adenosine A2A receptor
- aCSF:
-
Artificial cerebrospinal fluid
- AD:
-
Alzheimer’s disease
- AMPAR:
-
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- AP5:
-
DL-2-Amino-5-phosphonopentanoic acid
- ARs:
-
Adenosine receptors
- CA1 :
-
Cornu Ammonis 1
- Ca2+ :
-
Calcium ion
- CNQX:
-
6-Cyano-7-nitroquinoxaline-2,3-dione disodium salt hydrate
- CNS :
-
Central nervous system
- DIC:
-
Day in culture
- DIC-IR:
-
Differential interference contrast-infrared
- DPCPX:
-
8-Cyclopentyl-1,3-dipropylxanthine
- EDTA:
-
Ethylenediaminetetraacetic acid
- EPSCs:
-
Excitatory post-synaptic currents
- F340/380:
-
Ratio from excitation wavelength 340 nm and 380 nm
- FBS:
-
Fetal bovine serum
- fEPSP:
-
Field excitatory post-synaptic potential
- GABA:
-
Gamma-aminobutyric acid
- HBSS:
-
Hank’s balanced salt solution
- LTD:
-
Long-term depression
- LTP:
-
Long-term potentiation
- mGluR:
-
Metabotropic glutamate receptors
- NMDA:
-
N-Methyl-d-aspartate
- NMDAR:
-
N-Methyl-d-aspartate receptor
- NMDAR-EPSCs:
-
N-Methyl-d-aspartate receptor excitatory post-synaptic potential
- PDL:
-
Poly-d-lysine
- PPF:
-
Paired-pulse facilitation
- S.E.M.:
-
Standard error of the mean
References
Meeusen R, Roelands B, Spriet LL (2013) Caffeine, exercise and the brain. Nestle Nutr Inst work Ser 76:1–12. https://doi.org/10.1159/000350223
Cappelletti S, Piacentino D, Sani G, Aromatario M (2015) Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol 13:71–88. /https://doi.org/10.2174/1570159X13666141210215655
Hackett PH (2010) Caffeine at high altitude: java at base cAMP. High alt med biol 11:13–17. https://doi.org/10.1089/ham.2009.1077
Mitchell DC, Hockenberry J, Teplansky R, Hartman TJ (2015) Assessing dietary exposure to caffeine from beverages in the U.S. population using brand-specific versus category-specific caffeine values. Food Chem Toxicol 80:247–252. https://doi.org/10.1016/j.fct.2015.03.024
Poole RL, Braak D, Gould TJ (2016) Concentration- and age-dependent effects of chronic caffeine on contextual fear conditioning in C57BL/6J mice. Behav brain res 298:69–77. https://doi.org/10.1016/j.bbr.2015.03.045
Wikoff D, Welsh BT, Henderson R et al (2017) Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxicol 109:585–648. https://doi.org/10.1016/j.fct.2017.04.002
Ludden AB, O’Brien EM, Pasch KE (2017) Beliefs, behaviors, and contexts of adolescent caffeine use: a focus group study. Subst use misuse 52:1207–1218. https://doi.org/10.1080/10826084.2017.1302957
Nehlig A (2016) Effects of coffee/caffeine on brain health and disease: what should I tell my patients? Pr Neurol 16:89–95. https://doi.org/10.1136/practneurol-2015-001162
Verster JC (2014) Caffeine consumption in children, adolescents and adults. Curr Drug Abus Rev 7:133–134
Williams M, Jarvis MF (1988) Adenosine antagonists as potential therapeutic agents. Pharmacol Biochem Behav 29:433–441
Daly JW, Fredholm BB (1998) Caffeine--an atypical drug of dependence. Drug alcohol depend 51:199–206. https://doi.org/10.1016/s0376-8716(98)00077-5
Fredholm BB, Battig K, Holmen J et al (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51:83–133
Ribeiro JA, Sebastiao AM (2010) Caffeine and adenosine. J Alzheimers Dis 20(Suppl 1):S3–S15. https://doi.org/10.3233/JAD-2010-1379
Fredholm BB, Yang J, Wang Y (2017) Low, but not high, dose caffeine is a readily available probe for adenosine actions. Mol asp med 55:20–25. https://doi.org/10.1016/j.mam.2016.11.011
Dunwiddie TV, Hoffer BJ, Fredholm BB (1981) Alkylxanthines elevate hippocampal excitability - evidence for a role of endogenous adenosine. Naunyn Schmiedebergs arch Pharmacol 316:326–330. https://doi.org/10.1007/BF00501365
Sheth S, Brito R, Mukherjea D et al (2014) Adenosine receptors: expression, function and regulation. Int J Mol Sci 15:2024–2052. https://doi.org/10.3390/ijms15022024
Sebastião AM, Ribeiro JA (2009) Adenosine receptors and the central nervous system. Handb Exp Pharmacol 193:471–534
Ribeiro JA, Sebastiao AM, de Mendonca A (2002) Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol 68:377–392
Fredholm BB, IJ AP, Jacobson KA et al (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552
Sebastiao AM, Ribeiro JA (1996) Adenosine A2 receptor-mediated excitatory actions on the nervous system. Prog Neurobiol 48:167–189. https://doi.org/10.1016/0301-0082(95)00035-6
Li XX, Nomura T, Aihara H, Nishizaki T (2001) Adenosine enhances glial glutamate efflux via A2a adenosine receptors. Life Sci 68:1343–1350
Rodrigues RJ, Alfaro TM, Rebola N et al (2005) Co-localization and functional interaction between adenosine a(2A) and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem 92:433–441. https://doi.org/10.1111/j.1471-4159.2004.02887.x
Rebola N, Canas PM, Oliveira CR, Cunha RA (2005) Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience 132:893–903. /https://doi.org/10.1016/j.neuroscience.2005.01.014
Mouro FM, Rombo DM, Dias RB, et al (2018) Adenosine A2A receptors facilitate synaptic NMDA currents in CA1 pyramidal neurons. Br J Pharmacol 175:4386–4397. /https://doi.org/10.1111/bph.14497
Arendash GW, Cao C (2010) Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers dis 20 Suppl 1:S117-26. /https://doi.org/10.3233/JAD-2010-091249
de Mendonca A, Cunha RA (2010) Therapeutic opportunities for caffeine in Alzheimer’s disease and other neurodegenerative disorders. J Alzheimers dis 20 Suppl 1:S1-2. /https://doi.org/10.3233/JAD-2010-01420
Alhaider IA, Aleisa AM, Tran TT, Alzoubi KH, Alkadhi KA (2010) Chronic caffeine treatment prevents sleep deprivation-induced impairment of cognitive function and synaptic plasticity. Sleep 33:437–444
Costenla AR, Cunha RA, de Mendonca A (2010) Caffeine, adenosine receptors, and synaptic plasticity. J Alzheimers dis 20(Suppl 1):S25–S34. https://doi.org/10.3233/JAD-2010-091384
Faivre E, Coelho JE, Zornbach K, et al (2018) Beneficial effect of a selective adenosine A2A receptor antagonist in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Front Mol Neurosci 11:235. /https://doi.org/10.3389/fnmol.2018.00235
Lopes JP, Pliassova A, Cunha RA (2019) The physiological effects of caffeine on synaptic transmission and plasticity in the mouse hippocampus selectively depend on adenosine A1 and A2A receptors. Biochem Pharmacol 166:313–321. https://doi.org/10.1016/j.bcp.2019.06.008
Dore K, Stein IS, Brock JA et al (2017) Unconventional NMDA receptor signaling. J Neurosci 37:10800–10807. https://doi.org/10.1523/JNEUROSCI.1825-17.2017
Vyklicky V, Korinek M, Smejkalova T et al (2014) Structure, function, and pharmacology of NMDA receptor channels. Physiol Res 63(Suppl 1):S191–S203
de Mendonca A, Sebastiao AM, Ribeiro JA (1995) Inhibition of NMDA receptor-mediated currents in isolated rat hippocampal neurones by adenosine A1 receptor activation. Neuroreport 6:1097–1100
Deng Q, Terunuma M, Fellin T, et al (2011) Astrocytic activation of A1 receptors regulates the surface expression of NMDA receptors through a Src kinase dependent pathway. Glia 59:1084–1093. /https://doi.org/10.1002/glia.21181
Rebola N, Lujan R, Cunha RA, Mulle C (2008) Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 57:121–134. /https://doi.org/10.1016/j.neuron.2007.11.023
Sarantis K, Tsiamaki E, Kouvaros S, et al (2015) Adenosine a(2) a receptors permit mGluR5-evoked tyrosine phosphorylation of NR2B (Tyr1472) in rat hippocampus: a possible key mechanism in NMDA receptor modulation. J Neurochem 135:714–726. /https://doi.org/10.1111/jnc.13291
Kouvaros S, Papatheodoropoulos C (2016) Major dorsoventral differences in the modulation of the local CA1 hippocampal network by NMDA, mGlu5, adenosine A2A and cannabinoid CB1 receptors. Neuroscience 317:47–64. /https://doi.org/10.1016/j.neuroscience.2015.12.059
Tebano MT, Martire A, Rebola N, et al (2005) Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-d-aspartate effects. J Neurochem 95:1188–1200. /https://doi.org/10.1111/j.1471-4159.2005.03455.x
Temido-Ferreira M, Ferreira DG, Batalha VL, et al (2018) Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Mol psychiatry 1–25. /https://doi.org/10.1038/s41380-018-0110-9
Krania P, Dimou E, Bantouna M, et al (2018) Adenosine a 2A receptors are required for glutamate mGluR5- and dopamine D1 receptor-evoked ERK1/2 phosphorylation in rat hippocampus: involvement of NMDA receptor. J Neurochem 145:217–231. /https://doi.org/10.1111/jnc.14268
Sebastiao AM, de Mendonca A, Moreira T, Ribeiro JA (2001) Activation of synaptic NMDA receptors by action potential-dependent release of transmitter during hypoxia impairs recovery of synaptic transmission on reoxygenation. J Neurosci 21:8564–8571
Zhang DS, Ren LM, Zhang L (2004) [Relation between adenosine A1 receptor and NMDA receptor on synaptic transmission in dentate gyrus of hippocampus]. Yao Xue Xue Bao 39:245–249
Costenla AR, Diogenes MJ, Canas PM et al (2011) Enhanced role of adenosine a(2A) receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur J Neurosci 34:12–21. https://doi.org/10.1111/j.1460-9568.2011.07719.x
Cunha RA (2016) How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem 139:1019–1055. https://doi.org/10.1111/jnc.13724
Ribeiro FF, Xapelli S, Miranda-Lourenco C et al (2016) Purine nucleosides in neuroregeneration and neuroprotection. Neuropharmacology 104:226–242. https://doi.org/10.1016/j.neuropharm.2015.11.006
Dong ZS, Cao ZP, Shang YJ et al (2019) Neuroprotection of cordycepin in NMDA-induced excitotoxicity by modulating adenosine A1 receptors. Eur J Pharmacol 853:325–335. https://doi.org/10.1016/j.ejphar.2019.04.015
Jeronimo-Santos A, Vaz SH, Parreira S et al (2015) Dysregulation of TrkB receptors and BDNF function by amyloid-beta peptide is mediated by Calpain. Cereb cortex 25:3107–3121. https://doi.org/10.1093/cercor/bhu105
Ferreira DG, Temido-Ferreira M, Miranda H V, et al (2017) Alpha-synuclein interacts with PrP(C) to induce cognitive impairment through mGluR5 and NMDAR2B. Nat Neurosci 20:1569–1579. /https://doi.org/10.1038/nn.4648
Horvat A, Zorec R, Vardjan N (2016) Adrenergic stimulation of single rat astrocytes results in distinct temporal changes in intracellular Ca(2+) and cAMP-dependent PKA responses. Cell calcium 59:156–163. https://doi.org/10.1016/j.ceca.2016.01.002
Dunwiddie TV, Masino SA (2001) The role and regulation of adenosine in the central nervous system. Annu rev Neurosci 24:31–55. https://doi.org/10.1146/annurev.neuro.24.1.31
Corradetti R, Lo Conte G, Moroni F et al (1984) Adenosine decreases aspartate and glutamate release from rat hippocampal slices. Eur J Pharmacol 104:19–26. https://doi.org/10.1016/0014-2999(84)90364-9
Creager R, Dunwiddie T, Lynch G (1980) Paired-pulse and frequency facilitation in the CA1 region of the in vitro rat hippocampus. J Physiol 299:409–424. https://doi.org/10.1113/jphysiol.1980.sp013133
Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu rev Physiol 64:355–405. https://doi.org/10.1146/annurev.physiol.64.092501.114547
Ongini E, Dionisotti S, Gessi S et al (1999) Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn Schmiedebergs arch Pharmacol 359:7–10. https://doi.org/10.1007/pl00005326
Ongini E, Monopoli A, Cacciari B, Baraldi PG (2001) Selective adenosine A2A receptor antagonists. Farmaco 56:87–90. https://doi.org/10.1016/s0014-827x(01)01024-2
Zocchi C, Ongini E, Conti A, Monopoli A, Negretti A, Baraldi PG, Dionisotti S (1996) The non-xanthine heterocyclic compound SCH 58261 is a new potent and selective A2a adenosine receptor antagonist. J Pharmacol Exp Ther 276:398–404
Iacobucci GJ, Popescu GK (2017) NMDA receptors: linking physiological output to biophysical operation. Nat rev Neurosci 18:236–249. https://doi.org/10.1038/nrn.2017.24
Sebastião AM, Ribeiro JA (2015) Neuromodulation and metamodulation by adenosine: impact and subtleties upon synaptic plasticity regulation. Brain res 1621:102–113. https://doi.org/10.1016/j.brainres.2014.11.008
Daly JW, Fredholm BB (1998) Caffeine--an atypical drug of dependence. Drug Alcohol Depend 51:199–206
Simons SB, Caruana DA, Zhao M, Dudek SM (2012) Caffeine-induced synaptic potentiation in hippocampal CA2 neurons. Nat Neurosci 15:23–25. https://doi.org/10.1038/nn.2962
Martín ED, Buño W (2003) Caffeine-mediated presynaptic long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurophysiol 89:3029–3038. https://doi.org/10.1152/jn.00601.2002
McKinney RA (2010) Excitatory amino acid involvement in dendritic spine formation, maintenance and remodelling. J Physiol 588:107–116. https://doi.org/10.1113/jphysiol.2009.178905
Manent JB, Represa A (2007) Neurotransmitters and brain maturation: early paracrine actions of GABA and glutamate modulate neuronal migration. Neuroscientist 13:268–279. https://doi.org/10.1177/1073858406298918
Schlett K (2006) Glutamate as a modulator of embryonic and adult neurogenesis. Curr Top Med Chem 6:949–960
Traynelis SF, Wollmuth LP, McBain CJ et al (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol rev 62:405–496. https://doi.org/10.1124/pr.109.002451
Allene C, Cossart R (2010) Early NMDA receptor-driven waves of activity in the developing neocortex: physiological or pathological network oscillations? J Physiol 588:83–91. https://doi.org/10.1113/jphysiol.2009.178798
Pereira-Figueiredo D, Brito R, Araújo DSM, et al (2020) Caffeine exposure ameliorates acute ischemic cell death in avian developing retina. Purinergic signal 16:41–59. /https://doi.org/10.1007/s11302-020-09687-1
Silva CG, Métin C, Fazeli W, et al (2013) Adenosine receptor antagonists including caffeine alter fetal brain development in mice. Sci Transl med 5:197ra104-197ra104. /https://doi.org/10.1126/scitranslmed.3006258
Diógenes MJ, Assaife-Lopes N, Pinto-Duarte A et al (2007) Influence of age on BDNF modulation of hippocampal synaptic transmission: interplay with adenosine A2A receptors. Hippocampus 17:577–585. https://doi.org/10.1002/hipo.20294
Dumas TC, Foster TC (1998) Late developmental changes in the ability of adenosine A1 receptors to regulate synaptic transmission in the hippocampus. Brain Res Dev Brain Res 105:137–139
Coppi E, Pugliese AM, Stephan H et al (2007) Role of P2 purinergic receptors in synaptic transmission under normoxic and ischaemic conditions in the CA1 region of rat hippocampal slices. Purinergic signal 3:203–219. https://doi.org/10.1007/s11302-006-9049-4
Fusco I, Cherchi F, Catarzi D et al (2019) Functional characterization of a novel adenosine A2B receptor agonist on short-term plasticity and synaptic inhibition during oxygen and glucose deprivation in the rat CA1 hippocampus. Brain res bull 151:174–180. https://doi.org/10.1016/j.brainresbull.2019.05.018
Markram H, Segal M (1992) The inositol 1,4,5-trisphosphate pathway mediates cholinergic potentiation of rat hippocampal neuronal responses to NMDA. J Physiol 447:513–533. https://doi.org/10.1113/jphysiol.1992.sp019015
Clark KA, Randall AD, Collingridge GL (1994) A comparison of paired-pulsed facilitation of AMPA and NMDA receptor-mediated excitatory postsynaptic currents in the hippocampus. Exp brain res 101:272–278. https://doi.org/10.1007/bf00228747
Fontinha BM, Delgado-García JM, Madroñal N et al (2009) Adenosine a(2A) receptor modulation of hippocampal CA3-CA1 synapse plasticity during associative learning in behaving mice. Neuropsychopharmacology 34:1865–1874. https://doi.org/10.1038/npp.2009.8
Pedata F, Pepeu G, Spignoli G (1984) Biphasic effect of methylxanthines on acetylcholine release from electrically-stimulated brain slices. Br J Pharmacol 83:69–73. https://doi.org/10.1111/j.1476-5381.1984.tb10120.x
Spignoli G, Pedata F, Pepeu G (1984) A1 and A2 adenosine receptors modulate acetylcholine release from brain slices. Eur J Pharmacol 97:341–342. https://doi.org/10.1016/0014-2999(84)90475-8
Acknowledgments
The authors in Portugal are members of an EU twinning action (Project grant 952455). While revising this work, the authors want to make a special tribute to Geoffrey Burnstock for his great contributions to science, for defending and enlarging the knowledge of the purinergic field to the very end of his life, for his friendship and trust even in very difficult circumstances, which will never be forgotten. Thanks Geoff. Your legacy will last forever. We will miss your loud voice and laugh.
Code availability
Not applicable
Funding
Robertta Silva Martins was in receipt of a fellowship from Fundação de Amparo à Pesquisa do Estado do Rio de Jan eiro–FAPERJ, Brasil, Bolsa de Doutorado Sanduíche Europa/Oriente (grant number E-26/201.599/2018). The work was supported by Fundação para a Ciência e Tecnologia, Portugal (project grant PTDC/MED-FAR/30933/2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
All experimental procedures were performed according to European Community Guidelines (Directive 2010/63/EU) and the Portuguese Law (DL 113/2013) for animal care for research purposes and were approved by the “Instituto de Medicina Molecular” Internal Committee and the Portuguese Animal Ethics Committee - Direcção Geral de Veterinária.
Consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martins, R.S., Rombo, D.M., Gonçalves-Ribeiro, J. et al. Caffeine has a dual influence on NMDA receptor–mediated glutamatergic transmission at the hippocampus. Purinergic Signalling 16, 503–518 (2020). https://doi.org/10.1007/s11302-020-09724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-020-09724-z