Abstract
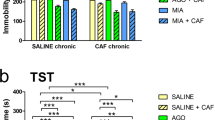
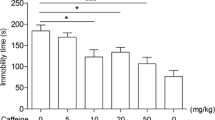
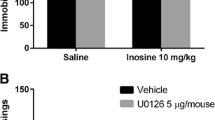
The benefits of creatine supplementation have been reported in a broad range of central nervous systems diseases, including depression. A previous study from our group demonstrated that creatine produces an antidepressant-like effect in the tail suspension test (TST), a predictive model of antidepressant activity. Since depression is associated with a dysfunction of the adenosinergic system, we investigated the involvement of adenosine A1 and A2A receptors in the antidepressant-like effect of creatine in the TST. The anti-immobility effect of creatine (1 mg/kg, po) or ketamine (a fast-acting antidepressant, 1 mg/kg, ip) in the TST was prevented by pretreatment of mice with caffeine (3 mg/kg, ip, nonselective adenosine receptor antagonist), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) (2 mg/kg, ip, selective adenosine A1 receptor antagonist), and 4-(2-[7-amino-2-{2-furyl}{1,2,4}triazolo-{2,3-a}{1,3,5}triazin-5-yl-amino]ethyl)-phenol (ZM241385) (1 mg/kg, ip, selective adenosine A2A receptor antagonist). In addition, the combined administration of subeffective doses of creatine and adenosine (0.1 mg/kg, ip, nonselective adenosine receptor agonist) or inosine (0.1 mg/kg, ip, nucleoside formed by the breakdown of adenosine) reduced immobility time in the TST. Moreover, the administration of subeffective doses of creatine or ketamine combined with N-6-cyclohexyladenosine (CHA) (0.05 mg/kg, ip, selective adenosine A1 receptor agonist), N-6-[2-(3,5-dimethoxyphenyl)-2-(methylphenyl)ethyl]adenosine (DPMA) (0.1 mg/kg, ip, selective adenosine A2A receptor agonist), or dipyridamole (0.1 μg/mouse, icv, adenosine transporter inhibitor) produced a synergistic antidepressant-like effect in the TST. These results indicate that creatine, similarly to ketamine, exhibits antidepressant-like effect in the TST probably mediated by the activation of both adenosine A1 and A2A receptors, further reinforcing the potential of targeting the purinergic system to the management of mood disorders.







Similar content being viewed by others
Abbreviations
- CHA:
-
N6-cyclohexyladenosine
- DPCPX:
-
8-cyclopentyl-1,3-dipropylxanthine
- DPMA:
-
N6-[2-(3,5-dimethoxyphenyl)-2-(methylphenyl)ethyl]adenosine
- TST:
-
Tail suspension test
- icv :
-
Intracerebroventricular
- ip :
-
Intraperitoneal
- po :
-
Per os
- ZM241385:
-
4-(2-[7-amino-2-{2-furyl}{1,2,4}triazolo-{2,3-a}{1,3,5}triazin-5-yl-amino]ethyl)-phenol
References
Andlin-Sobocki P, Jonsson B, Wittchen HU, Olesen J (2005) Cost of disorders of the brain in Europe. Eur J Neurol 12(Suppl 1):1–27
Andlin-Sobocki P, Wittchen HU (2005) Cost of affective disorders in Europe. Eur J Neurol 12(Suppl 1):34–38
Kessler D, Sharp D, Lewis G (2005) Screening for depression in primary care. Br J Gen Pract 55(518):659–660
Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Ustun TB, Wang PS (2009) The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiologia e Psichiatria Sociale 18(1):23–33
Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ustun TB (2009) The WHO World Mental Health (WMH) Surveys. Psychiatrie 6(1):5–9
Viana MC, Gruber MJ, Shahly V, Alhamzawi A, Alonso J, Andrade LH, Angermeyer MC, Benjet C, Bruffaerts R, Caldas-de-Almeida JM, Girolamo G, Jonge P, Ferry F, Florescu S, Gureje O, Haro JM, Hinkov H, Hu C, Karam EG, Lepine JP, Levinson D, Posada-Villa J, Sampson NA, Kessler RC (2013) Family burden related to mental and physical disorders in the world: results from the WHO World Mental Health (WMH) surveys. Rev Bras Psiquiatr 35(2):115–125
Price RB, Nock MK, Charney DS, Mathew SJ (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66(5):522–526
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475(7354):91–95
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47(4):351–354
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329(5994):959–964
Strakowski SM (2012) Bioenergetics for depression: something different for depression. Am J Psychiatry 169(9):891–893
Assis LC, Rezin GT, Comim CM, Valvassori SS, Jeremias IC, Zugno AI, Quevedo J, Streck EL (2009) Effect of acute administration of ketamine and imipramine on creatine kinase activity in the brain of rats. Rev Bras Psiquiatr 31(3):247–252
Wallimann T, Schnyder T, Schlegel J, Wyss M, Wegmann G, Rossi AM, Hemmer W, Eppenberger HM, Quest AF (1989) Subcellular compartmentation of creatine kinase isoenzymes, regulation of CK and octameric structure of mitochondrial CK: important aspects of the phosphoryl-creatine circuit. Prog Clin Biol Res 315:159–176
Hemmer W, Wallimann T (1993) Functional aspects of creatine kinase in brain. Dev Neurosci 15(3–5):249–260
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281(Pt 1):21–40
Cunha MP, Machado DG, Capra JC, Jacinto J, Bettio LE, Rodrigues AL (2012) Antidepressant-like effect of creatine in mice involves dopaminergic activation. J Psychopharmacol 26(11):1489–1501
Cunha MP, Pazini FL, Oliveira A, Bettio LE, Rosa JM, Machado DG, Rodrigues AL (2013) The activation of alpha1-adrenoceptors is implicated in the antidepressant-like effect of creatine in the tail suspension test. Prog Neuro-Psychopharmacol Biol Psychiatry 44:39–50
Cunha MP, Pazini FL, Oliveira A, Machado DG, Rodrigues AL (2013) Evidence for the involvement of 5-HT1A receptor in the acute antidepressant-like effect of creatine in mice. Brain Res Bull 95:61–69
Lyoo IK, Yoon S, Kim TS, Hwang J, Kim JE, Won W, Bae S, Renshaw PF (2012) A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. Am J Psychiatry 169(9):937–945
Roitman S, Green T, Osher Y, Karni N, Levine J (2007) Creatine monohydrate in resistant depression: a preliminary study. Bipolar Disord 9(7):754–758
Kondo DG, Sung YH, Hellem TL, Fiedler KK, Shi X, Jeong EK, Renshaw PF (2011) Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: a 31-phosphorus magnetic resonance spectroscopy study. J Affect Disord 135(1–3):354–361
Cunha MP, Pazini FL, Ludka FK, Rosa JM, Oliveira A, Budni J, Ramos-Hryb AB, Lieberknecht V, Bettio LE, Martin-de-Saavedra MD, Lopez MG, Tasca CI, Rodrigues AL (2015) The modulation of NMDA receptors and L-arginine/nitric oxide pathway is implicated in the anti-immobility effect of creatine in the tail suspension test. Amino Acids. doi:10.1007/s00726-014-1910-0
Deldicque L, Theisen D, Bertrand L, Hespel P, Hue L, Francaux M (2007) Creatine enhances differentiation of myogenic C2C12 cells by activating both p38 and Akt/PKB pathways. Am J Physiol Cell Physiol 293(4):C1263–C1271
Almeida LS, Salomons GS, Hogenboom F, Jakobs C, Schoffelmeer AN (2006) Exocytotic release of creatine in rat brain. Synapse 60(2):118–123
Cunha MP, Budni J, Pazini FL, Oliveira A, Rosa JM, Lopes MW, Leal RB, Rodrigues AL (2014) Involvement of PKA, PKC, CAMK-II and MEK1/2 in the acute antidepressant-like effect of creatine in mice. Pharmacol Rep 66(4):653–659
Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM (2005) Adenosine and brain function. Int Rev Neurobiol 63:191–270
Fredholm BB, Chen JF, Masino SA, Vaugeois JM (2005) Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol 45:385–412
Cunha RA (2008) Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem Int 52(1–2):65–72
Cunha RA, Ferre S, Vaugeois JM, Chen JF (2008) Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des 14(15):1512–1524
Blum D, Hourez R, Galas MC, Popoli P, Schiffmann SN (2003) Adenosine receptors and Huntington’s disease: implications for pathogenesis and therapeutics. Lancet Neurol 2(6):366–374
El Yacoubi M, Ledent C, Parmentier M, Bertorelli R, Ongini E, Costentin J, Vaugeois JM (2001) Adenosine A2A receptor antagonists are potential antidepressants: evidence based on pharmacology and A2A receptor knockout mice. Br J Pharmacol 134(1):68–77
Kaster MP, Rosa AO, Rosso MM, Goulart EC, Santos AR, Rodrigues AL (2004) Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci Lett 355(1–2):21–24
Lara DR, Dall’Igna OP, Ghisolfi ES, Brunstein MG (2006) Involvement of adenosine in the neurobiology of schizophrenia and its therapeutic implications. Prog Neuro-Psychopharmacol Biol Psychiatry 30(4):617–629
Simola N, Fenu S, Baraldi PG, Tabrizi MA, Morelli M (2006) Dopamine and adenosine receptor interaction as basis for the treatment of Parkinson’s disease. J Neurol Sci 248(1–2):48–52
Laursen SE, Belknap JK (1986) Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods 16(4):355–357
Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates, 1st edn. Academic, San Diego
Kaster MP, Budni J, Gazal M, Cunha MP, Santos AR, Rodrigues AL (2013) The antidepressant-like effect of inosine in the FST is associated with both adenosine A1 and A2A receptors. Purinergic Signal 9(3):481–486
Lobato KR, Binfare RW, Budni J, Rosa AO, Santos AR, Rodrigues AL (2008) Involvement of the adenosine A1 and A2A receptors in the antidepressant-like effect of zinc in the forced swimming test. Prog Neuro-Psychopharmacol Biol Psychiatry 32(4):994–999
Cunha MP, Machado DG, Bettio LE, Capra JC, Rodrigues AL (2008) Interaction of zinc with antidepressants in the tail suspension test. Prog Neuro-Psychopharmacol Biol Psychiatry 32(8):1913–1920
Ludka FK, Zomkowski AD, Cunha MP, Dal-Cim T, Zeni AL, Rodrigues AL, Tasca CI (2013) Acute atorvastatin treatment exerts antidepressant-like effect in mice via the L-arginine-nitric oxide-cyclic guanosine monophosphate pathway and increases BDNF levels. Eur Neuropsychopharmacol 23(5):400–412
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85(3):367–370
Machado DG, Bettio LE, Cunha MP, Capra JC, Dalmarco JB, Pizzolatti MG, Rodrigues AL (2009) Antidepressant-like effect of the extract of Rosmarinus officinalis in mice: involvement of the monoaminergic system. Prog Neuro-Psychopharmacol Biol Psychiatry 33(4):642–650
Kohrs R, Durieux ME (1998) Ketamine: teaching an old drug new tricks. Anesth Analg 87(5):1186–1193
Monteggia LM, Zarate C Jr (2015) Antidepressant actions of ketamine: from molecular mechanisms to clinical practice. Curr Opin Neurobiol 30C:139–143
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63(8):856–864
Abdallah CG, Sanacora G, Duman RS, Krystal JH (2014) Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. doi:10.1146/annurev-med-053013-062946
Ross AE, Venton BJ (2015) Adenosine transiently modulates stimulated dopamine release in the caudate-putamen via A1 receptors. J Neurochem 132(1):51–60
Ferreira SG, Goncalves FQ, Marques JM, Tome AR, Rodrigues RJ, Nunes-Correia I, Ledent C, Harkany T, Venance L, Cunha RA, Kofalvi A (2015) Presynaptic adenosine A2A receptors dampen cannabinoid CB1 receptor-mediated inhibition of corticostriatal glutamatergic transmission. Br J Pharmacol 172(4):1074–1086. doi:10.1111/bph.12970
Gerevich Z, Wirkner K, Illes P (2002) Adenosine A2A receptors inhibit the N-methyl-D-aspartate component of excitatory synaptic currents in rat striatal neurons. Eur J Pharmacol 451(2):161–164
Okada M, Nutt DJ, Murakami T, Zhu G, Kamata A, Kawata Y, Kaneko S (2001) Adenosine receptor subtypes modulate two major functional pathways for hippocampal serotonin release. J Neurosci 21(2):628–640
Dias RB, Rombo DM, Ribeiro JA, Henley JM, Sebastiao AM (2013) Adenosine: setting the stage for plasticity. Trends Neurosci 36(4):248–257
Kaster MP, Machado DG, Santos AR, Rodrigues AL (2012) Involvement of NMDA receptors in the antidepressant-like action of adenosine. Pharmacol Rep 64(3):706–713
El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM (2000) The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A(2A) adenosine receptor antagonists. Psychopharmacology 148(2):153–163
Jain N, Kemp N, Adeyemo O, Buchanan P, Stone TW (1995) Anxiolytic activity of adenosine receptor activation in mice. Br J Pharmacol 116(3):2127–2133
Kaster MP, Santos AR, Rodrigues AL (2005) Involvement of 5-HT1A receptors in the antidepressant-like effect of adenosine in the mouse forced swimming test. Brain Res Bull 67(1–2):53–61
Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51(1):83–133
Uzbay TI, Kayir H, Ceyhan M (2007) Effects of tianeptine on onset time of pentylenetetrazole-induced seizures in mice: possible role of adenosine A1 receptors. Neuropsychopharmacology 32(2):412–416
Hines DJ, Schmitt LI, Hines RM, Moss SJ, Haydon PG (2013) Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl Psychiatry 3:e212
Marek GJ (2012) Activation of adenosine(1) receptors induces antidepressant-like, anti-impulsive effects on differential reinforcement of low-rate 72-s behavior in rats. J Pharmacol Exp Ther 341(2):564–570. doi:10.1124/jpet.112.191718
Muto J, Lee H, Lee H, Uwaya A, Park J, Nakajima S, Nagata K, Ohno M, Ohsawa I, Mikami T (2014) Oral administration of inosine produces antidepressant-like effects in mice. Sci Rep 4:4199
El Yacoubi M, Costentin J, Vaugeois JM (2003) Adenosine A2A receptors and depression. Neurology 61(11 Suppl 6):S82–S87
Yamada K, Kobayashi M, Kanda T (2014) Involvement of adenosine A2A receptors in depression and anxiety. Int Rev Neurobiol 119:373–393
Nomura A, Zhang M, Sakamoto T, Ishii Y, Morishima Y, Mochizuki M, Kimura T, Uchida Y, Sekizawa K (2003) Anti-inflammatory activity of creatine supplementation in endothelial cells in vitro. Br J Pharmacol 139(4):715–720
Mazar J, Rogachev B, Shaked G, Ziv NY, Czeiger D, Chaimovitz C, Zlotnik M, Mukmenev I, Byk G, Douvdevani A (2005) Involvement of adenosine in the antiinflammatory action of ketamine. Anesthesiology 102(6):1174–1181
Kulkarni SK, Mehta AK (1985) Purine nucleoside—mediated immobility in mice: reversal by antidepressants. Psychopharmacology 85(4):460–463
Blardi P, de Lalla A, Urso R, Auteri A, Dell’Erba A, Bossini L, Castrogiovanni P (2005) Activity of citalopram on adenosine and serotonin circulating levels in depressed patients. J Clin Psychopharmacol 25(3):262–266
Acknowledgments
This study was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), #308723/2013-9, Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES), NENASC project (PRONEX program CNPq/FAPESC).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cunha, M.P., Pazini, F.L., Rosa, J.M. et al. Creatine, similarly to ketamine, affords antidepressant-like effects in the tail suspension test via adenosine A1 and A2A receptor activation. Purinergic Signalling 11, 215–227 (2015). https://doi.org/10.1007/s11302-015-9446-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-015-9446-7