Abstract
Seed hoarding animals may distinctly impact individual fitness of plants as well as plant community dynamics in terms of differential seed consumption and seed dispersal. Addressing methodological challenges and constraints in understanding the role of seed dispersal mutualisms, we tested different seed tagging methods for relatively small seeds. We selected two study areas with dense ground vegetation, irregular terrain, and seed predator guilds with comparatively high dispersal and seed removal potential. We tested three different tagging methods, offering untagged and tagged seeds on experimental dishes: (1) wire threads with plastic flags fixed with solvent-free glue, (2) wire threads with plastic flags twisted around a beechnut, (3) and radio-transmitters fixed with solvent-free glue. Seed predators did not show any preferences for tagged or untagged seeds or for a certain tagging method at any point of time. Instead, both tagged seeds and untagged control seeds were removed from the dishes to a high degree (up to 100%) after 25–35 days. In both study areas, seeds with radio-transmitters experienced longer transport distances with a maximum of nearly 60 m and distances also differed between study areas. In one study area, higher portions of radio-tagged seeds could be recovered than for the flag-tagged seeds. Significantly more seeds tagged with radio-transmitters were cached than expected. Given the advantages and limitations of each approach, a combination of flag-tagging and radio-telemetry (not on the same seed) may be the best solution for tracking seed fate in dense vegetation or irregular terrain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed hoarding animals may distinctly impact individual fitness of plants as well as plant community dynamics via differential seed consumption and seed dispersal (Jensen 1985; Schnurr et al. 2004; Beckage and Clark 2005; Cousens et al. 2008; Beck and Vander Wall 2010; Wang et al. 2012; Wang and Ives 2017; Saastamoinen et al. 2018). For food hoarding animals, the investment of energy in hoarding increases fitness if predation pressure and food competition at the new location is lower and/or food is provided to progenies (Vander Wall and Jenkins 2003; Moore et al. 2007; Vander Wall 2010; Muñoz and Bonal 2011). Effects of food limitation on population dynamics are co-determined by the extent of cache recovery, which is either driven by olfactory or visual cues or by results of random cache searches of animals (Vander Wall 1990). Depending on animal species and its´ population densities on the one hand and on seeds´ sizes and nutrient contents on the other hand, transport distances of seeds may differ markedly (Jansen et al. 2002; Tamura and Hayashi 2008; Zhang et al. 2008).
According to the optimal cache theory (Stapanian and Smith 1978; Clarkson et al. 1986), caching effort balances costs of seed transport, food storing and cache loss, all of which are a function of distance and seed predator density. Depending on the location and burying depth, plants may benefit from incomplete recovery of caches because this inefficiency allows for establishment of seedlings even at sites, which had not been colonized by the plant species before (Guo and Werger 2001; Schnurr et al. 2002; Haas and Heske 2005; Beck and Vander Wall 2010). Hoarding behavior and the possible result of plant establishment at new sites can thus be an important part of forest regeneration dynamics. Many studies show that small rodents are a very efficient post-dispersal seed predator guild and therefore can have strong influence on tree population dynamics and tree species coexistence (e.g. Gómez et al. 2008; Tamura and Hayashi 2008).
The ultimate outcome of seed hoarding is seed dispersal to sites that provide higher likelihoods of successful establishment and thus higher fitness of plants (for unretrieved seeds) and increased fitness of seed hoarders through provision of resources (for retrieved and eaten seeds). Quantifying these components is far from trivial, particularly for the fitness response of long-lived plants. Characterizing the net outcome of dispersal around mother plants–the dispersal kernel (Nathan et al. 2012), does not allow to disentangle the effects of different dispersal agents. The multistep character of secondary dispersal (diplochory, Wenny 2000; Bonte et al. 2012) with multiple decisions between consumption and re-caching, limits the information provided by simple seed removal experiments.
The only option for quantifying both quality and quantity of the mutualistic interaction is to follow individual seed fates. This requires tagging of seeds and following them to their ultimate location. Basically, seed tagging studies should meet the following requirements (cf. Nathan et al. 2003; Lemke et al. 2009): (1) typical characteristics of seeds (weight, palatability, odor, germination capacity) should not or only marginally be modified; (2) marked seeds should be recovered easily within large areas including the margins of dispersal kernels; (3) seed marking should persist for relevant windows of time. In the past, a variety of methods have been applied, being based on visual (threads with color/tin tags, drilled holes), chemical (15N-urea enrichment, fingerprinting), or physical (fluorescence, radioactivity, magnetism, radio-transmittance) properties of marked individual seeds or germinants or of non-individually discriminated cohorts. Each method holds advantages and disadvantages: whereas large quantities of even small seeds marked with fluorescent powder, radioisotopes, or 15N-urea (Jensen 1985; Lemke et al. 2009; Beck and Vander Wall 2010; Carlo et al. 2013; Yi et al. 2014) might be tracked, individual dispersal histories remain undetected. Moreover, seeds with long dispersal distances face lower detection probabilities as search effort exponentially increases with search radius, resulting in underestimation of real seed dispersal distances (Hirsch et al. 2012). Finally, these methods might not be applied in case of dense ground vegetation (Wróbel and Zwolak 2013), in case of deep caching in the soil or due to legal restrictions for the use of radioactive matter.
Fingerprinting (Hasenkamp et al. 2011) might allow for an assignment of progenies to mother trees thus highlighting distances of successful dispersal but hardly for tracking long-distance dispersal. Telemetric transmitters partially overcome these problems, as they are not (distinctly) biased against long distance dispersal allowing for detection of seed caches without disturbing them (Hirsch et al. 2012). However, a trade-off between the life-time of batteries and the resulting weight of transmitters has to be found, both covering the ecologically important time span till germination and not affecting the behavior of potential seed dispersers/predators. Consequently, studies exploring zoochory with radio-transmitters hitherto typically addressed large-seeded plant species like oaks (Quercus sp.), walnuts (Juglans sp.), or palm species (Astrocaryum sp.; Soné and Kohno 1999; Tamura 2001; Sone et al. 2002; Pons and Pausas 2007; Tamura and Hayashi 2008; Suselbeek et al. 2013). Suselbeek et al. (2013) overcame the problem of significantly enhanced seed weights by using passive integrated transponders for acorns, but remarked, that these small transmitters have to be approached closely to be detected (as it is the case for tagging with magnets, metal or radioisotopes). Consequently, these transmitters are not suitable for tracking seeds under dense vegetation or in irregular terrain. For tracking the fate of beechnuts (Fagus sp.), plastic or tin tags, and fluorescent powder had been used in the past. In their review, Forget and Wenny (2005) considered beech seeds being too small for radio-transmitter tagging. However, weights of radio-transmitters have been minimized with technical progress within the last decade, suggesting seed tracking studies with beechnuts including radio-telemetry.
Although data on seed fate is presented in various studies, rigorous tests of the methods used are rather scarce (e.g., Xiao et al. 2006; Yi and Wang 2015). If available, they were frequently carried out in less complex ecosystems without dense understories and more homogenous vegetation structures. In order to overcome methodological challenges and constraints in understanding the role of seed dispersal mutualisms, we thus tested different seed tagging methods for relatively small seeds under field conditions that are potentially more challenging: we selected study areas with dense ground vegetation, irregular terrain and seed predator guilds with comparatively high dispersal and seed removal potential (> 30 m; Watts 1970; Nopp-Mayr et al. 2012).
We tested three different tagging methods: (1) wire threads with plastic flags fixed with flat washers and solvent-free glue to the beechnut, (2) wire threads with plastic flags twisted around a beechnut, (3) and radio-transmitters fixed with solvent-free glue to explore individual seed fate at ecologically relevant spatial scales in further steps. Thereby, we tested the following hypothesis:
-
1.
Recovery rates and final seed fate do not differ between differently tagged seeds.
-
2.
Total dispersal distances are not different for different tagging methods.
-
3.
Seed removal rates of tagged seeds does not differ from untagged seeds (= control).
Materials and methods
Study areas
We conducted the study at two different forest sites in two study areas, both representing typical habitats for Central European seed dispersing rodents (e.g. the Yellow-necked mouse Apodemus flavicollis and the bank vole Myodes glareolus).
Primary forest Rothwald
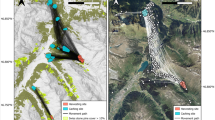
The first study area, the primary old-growth forest Rothwald is located in the Wilderness Area Dürrenstein (IUCN-Category I; 47°48′ to 47°45′N, 15°01′ to 15°07′E) and is part of the Northern Limestone Alps in the southwest of Lower Austria (Fig. 1). Mean annual temperature of the area is 3.7 °C and mean annual precipitation is 2200 mm (Roller cited in Zukrigl et al. 1963). The experimental plots were located at approx. 1000 m above sea level. The forest is dominated by European beech (Fagus sylvatica). Norway spruce (Picea abies) and Silver fir (Abies alba) are co-dominating in density and grow 10–15 m taller (up to approx. 60 m) than F. sylvatica, forming a two-layered canopy (for more details see Nopp-Mayr et al. 2015). Beside regeneration of trees, ground cover is dominated by Mercurialis perennis, Gallium sylvaticum, Helleborus niger, Vaccinium myrtillus etc. (Zukrigl et al. 1963; Ellmauer 2011). A typical characteristic of this natural mountain forest is a large amount of woody debris in all stages of decomposition.
Managed forest Schottenwald
The second study area, the managed forest Schottenwald, is located near the City of Vienna within the UNESCO Biosphere Reserve Wienerwald (47°56′ to 48°21′N, 16°22′ to 15°46′E, Fig. 1). Mean annual temperature of the area is 8 °C and mean annual precipitation is 837 mm. Geologically, the study area is located in the Flyschzone, consisting of sandstones and shales. The experimental plots were located at approx. 400 m a.s.l.. The forest is dominated by European beech (F. sylvatica), oak (Quercus robus, Q. petraea, Q. pubescens) and hornbeam (Carpinus betulus) (Brenner et al. 2015). Structural characteristics are typical for managed forests with little woody debris and some understory, mainly consisting of beech regeneration.
Experimental design
Caféteria experiments of Nopp-Mayr et al. (2012) were modified offering tagged seeds of European beech (F. sylvatica) to potential seed dispersers. Seeds of F. sylvatica have a length of approx. 2 cm and a thousand seed weight of 160–240 g (Burschel 1966; Rohmeder 1972). To avoid the disadvantages of drilling a hole through the seeds, we tested methods of beech seed tagging without drilling and the practicability of radio-transmitter application: Beech seeds were tagged with (1) a 25 cm wire thread (0.25 mm ø) and 1.5 × 3.0 cm plastic flag (white), fixed on a 8.5 mm flat washer which was attached on beechnuts with solvent-free glue in primary Forest Rothwald (Fig. 2a); (2) a 25 cm wire thread (0.25 mm ø) and 1.5 × 3.0 cm plastic flag (blue or pink), attached to the seed with a 25 cm wire thread (0.25 mm ø) without glue but with wire loop bonding in the managed forest Schottenwald (Fig. 2b); (3) a radio-transmitter (Biotrack PicoPip Ag392, 1 g weight, 1 cm ø) glued to the seeds with scentless glue in both study areas (Fig. 2a). Flat washers were not only used to better fix the wire threads on the seeds but also to allow for searching tagged seeds with a metal detector (Tesoro Compadre SE V2; Iida 2006; Moore et al. 2007; Cilles et al. 2016). Wire threads and flags had a weight of approx. 0.22 g and flat washers weighed about 0.23 g. Total weights of tagged seeds were 0.38–0.46 g for wire tagged seeds, 0.61–0.68 g for seeds equipped with wire tags and flat washers and 1.16–1.24 g for seeds tagged with radio-transmitters. All tagged seeds were numbered with black permanent marker for individual identification in the case of tag loss. All seeds were presented on green or brown plastic dishes (Nopp-Mayr et al. 2012).
In the primary forest Rothwald four canopy gaps were selected as experimental plots. At each gap six dishes were installed in October 2012. A total of 10 beech seeds were set on each dish using three seeds tagged with flag-tags, two with radio-transmitters, and five untagged seeds. In the managed forest Schottenwald three canopy gaps were chosen and nine dishes were set at each gap in May 2013. A total of 10 seeds of F. sylvatica were set on each dish using six seeds tagged with flags, one with a radio-transmitter, and three untagged seeds. Through logistic and budget constraints, the total number of radio-transmitters was slightly higher in the primary forest in 2012. Furthermore, we re-used transmitters from the primary forest on the managed forest study site. Altogether 510 seeds of European beech were experimentally exposed in the two study areas (nRothwald = 240, nSchottenwald = 270), whereby 309 seeds were either equipped with flag-tags or radio-transmitters, and 201 seeds were untagged (= control).
Tracking of seed fate started one day after seed exposure, on October 12th, 2012 in the primary forest Rothwald and on May 5th, 2013 in the managed forest Schottenwald, and was repeated 6 times (ntotal = 7) until day 27 in the primary forest and 11 times (ntotal = 12) until day 35 in the managed forest. We particularly considered seed removal and transfer on day 1, day 3 and day 9 or 10 after exposure, as these time windows covered the majority of initial seed removal events from experimental dishes in a previous study (Nopp-Mayr et al. 2012). Flag-tags were visually searched by starting at the center point (experimental dish) and walking slowly in a helix up to a radius of approx. 30 m around every dish (without a fixed time budget). In the primary forest Rothwald the visual search was supported by a metal detector. Radio-transmitters were tracked using a receiver (Biotrack SIKA radio tracking receiver) with antenna and headset by starting at the center point (experimental dish) and following the individual signal of every radio transmitter consecutively until the transmitter was found. As the range of the radio signals could reach up to 30 m, a larger area than a 30 m radius could be covered. When a flag-tagged seed was found outside the initial 30 m search radius, it was recorded as well. Searching was done by the same observer plus one or two assistants, whereby one person was searching with the Biotrack receiver and one person with the metal detector. As searching with the metal detector yielded only poor signals (due to dense vegetation structure and deeper caches) and did not work well we skipped searching with it after the second day. Each day the number of remaining seeds on the dishes was recorded, removed seeds were not substituted. We marked seed locations of cached seeds on day 27 in the primary forest (November 7th, 2012) and on day 35 in the managed forest (June 8th, 2013) with flags closely set to the caches, but not directly in the caches. We recorded the GPS coordinates of caches and determined survival and/or germination of the seeds in June 2013 for the primary forest and in August 2013 for the managed forest by depleting the caches.
Small mammal trapping
To monitor rodents´ populations as potential seed dispersers or predators, we live trapped small mammals’ populations in the primary forest Rothwald on two long-term grids (15 m spacing, 60 × 60 m grid, respectively) for three consecutive nights in June, August, and October 2012, respectively (Kempter and Nopp-Mayr 2013). We set two live traps per grid point, one Sherman Trap (LFA 7.6 × 8.9 × 22.9 cm; “LFA” is the product name, indicating its size; H.B. Sherman traps, Tallahassee, Florida, USA) and one Field Trip Trap with a nest box (tunnel 14 × 4 × 5 cm, nest box 14 × 8 × 6 cm; Alana Ecology, Bishops Castle, Shropshire, GB), placing in total 50 traps per grid. In the managed forest Schottenwald live trapping was done at each study plot (each canopy gap) for three consecutive nights in August 2013. We installed at each study plot 20 Sherman Traps (10 × LFA, 10× SFA 5.1 × 6.4 × 16.5 cm; “SFA” is the product name, indicating its size; H.B. Sherman traps, Tallahassee, Florida, USA) along two parallel transects (180 m long, distance between 20 m) with one Sherman Trap placed every 20 m. At both study areas, traps were baited with peanut butter, biscuit, and a piece of apple (Cody and Smallwood 1996). We identified catches following Niethammer and Krapp (1978; 1982), Gorbet and Ovenden (1980), and Görner and Hackethal (1988), marked the animals with a red waterproof marker at the tail-bottom and released them into the field at the point of capture. This marking was visible for one trapping period, respectively.
Statistical analyses
We compared the numbers of removed and remaining seeds per experimental dish for different tagging methods at fixed dates (day 1, 3, 9–10, 25–27) with χ2-tests (Zar 2010). Accordingly, final recovery rates of differently tagged seeds were analyzed with χ2-tests. We further compared the fate of seeds (consumed vs. cached) after 27–35 days among the type of tagging with χ2-tests. χ2-tests, looking for associations between different classes of categorical variables, were calculated for each matrix cell with the following formula:
with r being the rows and c the columns representing the levels of the categorical variables. fo denotes the numbers of observations in the matrix and fe the expected values, which are derived from the product of marginal row totals and marginal column totals divided by the grand total, respectively. χ2-statistics of each cell of the final χ2-matrix were used to infer their significance level: χ2-values > 3.8 indicate differences in observations with P < 0.05, χ2-values > 6.6 indicate differences with P < 0.01 and values > 10.8 differences with P < 0.001 (Zöfel 1992).
As dispersal distances significantly deviated from normality and transformations did not produce normality, we analyzed dispersal distances using non-parametric Mann–Whitney-U test. Thereby, we compared dispersal distances between tagging methods within each study area as well as dispersal distances of each tagging method between the study areas. As flag-tagged seeds outside the initial 30 m-search radius were only detected when we tracked seeds equipped with radio-transmitters, their recovery success was potentially driven by the larger detection range of radio-transmitters. To eliminate any effects of detectability on dispersal distances, we also run the comparisons of dispersal distances between tag types and study areas with truncated data. For this, we truncated dispersal distances of all seeds exceeding a 30-m distance.
We performed χ2-tests with the “stats” package in R (R Core Team 2017) and Mann–Whitney-U tests with IBM ® SPSS ® Statistics (Version 21).
Results
Comparisons of recovery rates between differently tagged seeds
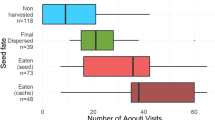
We did not observe significant differences in the recovery or loss between radio-transmitter-tagged and flag-tagged seeds in the primary forest Rothwald (cell matrix: recovery rate/loss rate vs. radio-transmitters/flag-tags, χ2 = 1.053, df = 1, P = 0.229, n = 84). In the managed forest Schottenwald, no single transmitter got completely lost (meaning a complete recovery failure). In single cases, transmitters were found without a seed attached to it. Recovery success responded to the flag-color with highest loss rates for white flags (27%), followed by the blue flags (14%) and lowest rates for the pink flags (8%). Of recovered seeds, a certain portion of seeds tagged with radio-transmitters could be located within a radius of 1 m (= class “located to the nearest meter”, Fig. 3a) without determination of exact coordinates in both areas, as these seeds had been cached under rocks, coarse woody debris etc. In the primary forest, 48% of seeds belonged to this recovery class, whereas only 8% of radio-tagged seeds could only be located to the nearest meter in the managed forest (Fig. 3a).
a Percentage of differently tagged beech seeds (radio-transmitters vs. flag-tags) finally recovered or lost in the primary and managed forest (“located to the nearest meter” indicates seeds, which could be located within a 1 m-radius); b Box-violin plot of observed transport distances (m) of beech seeds tagged with radio-transmitters or flag-tags within the two study areas (the violin represents the kernel density estimation)
Total dispersal distances for different tagging methods
In the primary forest Rothwald, the median of transport distances of seeds equipped with flag-tags was 1.4 m, for seeds with radio-transmitters 13.27 m; for the managed forest Schottenhof, median values were 1.8 m for the flag-tagged seeds and 7.6 m for the seeds with radio-transmitters. Thus, distances, at which tagged seeds were recovered, differed between the tagging methods: Beech seeds equipped with radio-transmitters were detected over significantly longer distances than seeds with flag-tags (primary forest: U = 926.0, n = 65, P < 0.001; managed forest: U = 2,313.5, n = 158, P = 0.001). Final distances in the two study areas differed as well: seeds equipped with radio-transmitters were located significantly farther away in the primary forest compared to the managed forest (U = 118.0, n = 48, P < 0.001; Fig. 3b). As for the truncated data (≤ 30 m search radius), we also observed differences in dispersal distances between the tagging methods. In both study areas, seeds with radio-transmitters were transported over larger distances than seeds with flag-tags (primary forest: U = 721.0, n = 60, P < 0.001; managed forest: U = 2,179.5, n = 157, P = 0.002). We further observed differences in truncated dispersal distances between the study areas for the seeds tagged with radio-transmitters with longer distances in the primary forest compared to the managed forest (U = 97.5, n = 42, P = 0.002). Truncated dispersal distances of flag-tagged seeds did not differ between the study areas (U = 2,878.0, n = 175, P = 0.644).
Effects of tagging on seed removal
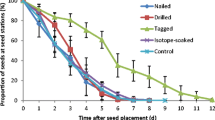
Both in the primary forest Rothwald and in the managed forest Schottenwald, the numbers of removed and remaining seeds did not significantly differ between tagged and untagged seeds and between tagging methods (flag-tags and radio-transmitters) on day 1, day 3 and day 9 after exposure (Fig. 4a, b; χ2-cell matrix: no tagging/flag-tagging/radio-transmitters vs. primary forest Rothwald/managed forest Schottenwald, P < 0.05 in all cases). By the end of the survey, the majority of seeds (92–100%) were removed from the experimental dishes (Fig. 4a, b) and there was no significant difference in the numbers of seeds of different tagging. Within the flag-tagged subsample, seeds tagged with pink or blue tags both disappeared to 100% from the dishes in the managed forest Schottenwald.
Removal ratio of flag-tagged seeds (white flags with flat washers), seeds with transmitters and untagged seeds during 27 days of exposure in the primary forest Rothwald (a), and removal ratio of flag-tagged seeds (pink and blue flags, no flat washers), seeds with transmitters and untagged seeds during 35 days of exposure in the managed forest Schottenwald (b)
Seed fate and seed treatment between differently tagged seeds
The fate of beech seeds (being either consumed or cached) at the end of seed tracking on day 27/35, significantly differed between seeds tagged with radio-transmitters or with flag-tags in both study areas (primary forest: χ2 = 35.019, df = 1, P < 0.001, n = 72; managed forest: χ2 = 20.975, df = 1, P < 0.001, n = 164). In both forest types, significantly more seeds tagged with radio-transmitters were cached than expected (P < 0.001). Additionally, in the primary forest significantly fewer seeds tagged with radio-transmitters were consumed (P < 0.05) and fewer seeds tagged with flag-tags were cached than expected (P < 0.01; Fig. 5). In the managed forest Schottenwald, numbers of recovered cached or consumed seeds did not significantly differ between the pink and blue flags (χ2 = 1.822, df = 1, P = 0.119).
However, none of the cached seeds survived or germinated till the final surveys (June 2013 in the primary forest and August 2013 in the managed forest) in both study areas.
Species composition of seed hoarding mammals
We detected six species of potential seed predators and/or dispersers in the primary forest Rothwald in 2012: The yellow-necked mouse A. flavicollis, the wood mouse A. sylvaticus, the bank vole Myodes glareolus, the common pine vole Microtus subterraneus, the edible dormouse Glis glis, and the common dormouse Muscardinus avellanarius. The majority (70%) of catches were M. glareolus and the remaining portion were mainly specimen of Apodemus sp. In contrast, we only caught the yellow-necked mouse and the bank vole in the managed forest Schottenwald in 2013, with three quarters of catches comprising of Apodemus sp. and one quarter of M. glareolus. The proxy for the density of the rodent populations, expressed in newly captured specimen (NC) per 100 trapping nights (TN), was 34 NC/100 TN for the primary forest in 2012 and 19 NC/100 TN for the managed forest in 2013.
Discussion
The effects of seed tagging methods on recovery rates and final seed fate
After a survey period of 27–35 days, both flag-tagging and radio-transmitters yielded comparatively high recovery rates in both study areas (72–100%, Table 1). Due to heterogeneously structured terrain and high coverage with coarse woody debris, about two-thirds of recovered radio-tagged seeds could be located within a 1 m radius with none of them being cached on microsites being suitable for following successful germination. Other studies using radio-transmitters for seed tagging yielded low loss rates as well or did not explicitly address portions of non-recovered seeds (Table 1). In one study area (Schottenwald), the recovery ratio of radio transmitter-tagged seeds was higher than that of flag-tagged seeds and we can thus not reject hypothesis 1. The color of flag tags resulted in different recovery rates, with best results for the pink flags and poorest results for the white flags. Thereby, not only the contrast between flag color and surrounding vegetation seemed to impact recovery success, but also the contrast to sunspots on the forest floor. We partially used flat washers to fix the wire thread of the flag tags on the seeds and to search them with a metal detector as done by Iida (2006), Moore et al. (2007), or Cilles et al. (2016). This method worked well in pretesting in smooth terrain but yielded only poor signals in the primary forest due to highly structured micro-relief and deeper caching of tagged seeds, e.g. under tree stumps. We thus skipped this searching method after the second day of the trial and focused on the visual search for flag-tagged seeds. In the managed forest Schottenwald, we thus developed a different method for fixing the flag-tags to the seeds, the wire-loop-bonding.
In both study areas, we could relocate all remaining cached seeds at their final locations, observing either their consumption by small mammals or germination failures.
The effects of seed tagging methods on dispersal distances
Whereas the initial removal of seeds from experimental dishes did not differ between the treatments of seeds, the distances of ultimate seed dispersal as well as dispersal distances within a 30-m radius significantly differed between tagging methods and we cannot reject hypothesis 2. In both study areas, seeds with a radio-transmitter experienced longer transport distances with a maximum of nearly 60 m in the primary forest Rothwald (Table 1, Fig. 3). There, the transport distances of radio-tracked seeds exceeded those observed in the managed forest Schottenwald. Consequently, the weight of radio-transmitters obviously had an impact seed predators´ dispersal behavior. However, identical radio-transmitters yielded significantly different transport distances between the study areas, most likely due to different caching decisions of small mammals according to a differing population status and species composition of small mammal communities (compare Lichti et al. 2017): In the primary forest Rothwald, we observed a peak in the number of newly captured individuals per 100 trapping night (being a proxy for high population densities), whereas in the managed forest Schottenwald, only moderate catch numbers occurred. Additionally, bank voles dominated the catches in the primary forest and Apodemus species formed only about 30% of catches, whereas the portions were converse in the managed forest. Apart from fairly differing habitat structures for small mammals between the two areas, also home ranges and territoriality differ between the species (Soné and Kohno 1999) and they further depend on population densities (Jensen 1985; Stradiotto et al. 2009). Thus, differences in transport distances between the study areas might be a result of these interacting factors (Morán-López et al. 2015; Sunyer et al. 2015). However, for the flag-tagged seeds, we found no significant differences in total transport distances between the seeds equipped with flat washers and wires or only with wires.
In our trial, we had to balance distance-biased recovery probabilities of tagged seeds with weight-biased behavior of seed hoarding small mammals. Flag-tagging of beechnuts (without fixing flat washers) modifies seed weights to a lower degree than fixing of radio-transmitters, but holds a higher risk of recovery failure at larger secondary dispersal distances, as search effort increases exponentially with increasing search radii (Hirsch et al. 2012). Consequently, the shorter transport distances of recovered flag-tagged seeds might be a result of detection failures at larger distances. Most published studies tracing the fate of fagaceous species and explicitly describing search radii, covered radii from 10 up to 30 m (e.g., Xiao et al. 2005; Yi et al. 2008; Yi and Wang 2015). Considering different medium- to large-seeded tree species, transport distances for seeds tagged with different methods (wire-plastic-tags, wire-tin-tags, and radio-transmitters), may differ distinctly (Table 1). For wire-plastic- or wire-tin-tags, mean transport distances of tagged seeds are up to 18 meters, with maximum values of more than 70 m (Table 1). However, searching efficiency for flag-tagged seeds at these distances is low and successful recovery may be anecdotic. Unfortunately, only few studies described searching protocols including search effort in terms of standardized search time or search radii. In our study, all recovered flag-tagged beechnuts were found within a 20 m radius (search radius = 30 m), but again, we cannot exclude the possibility that the lost flag-tagged seeds had been transported over distances larger than 20 m. For the truncated data set, where we only analyzed dispersal distances of seeds within a 30-m radius, also yielded differences between the tagging methods with farther dispersal of seeds tagged with radio-transmitters. However, focusing on small spatial scales potentially excludes effects of diplochory and long-distance seed dispersal.
The effects of seed tagging on seed removal rates
One basic assumption for appropriate tagging methods is that potential seed vectors do not discriminate between treatment (= tagged seeds) and control (= untagged seeds). We addressed seed predators´ behavior by comparing removal of tagged and untagged seeds from experimental dishes. A previous study gave evidence that small mammals were mainly responsible for seed removal from experimental dishes in the primary forest Rothwald (Nopp-Mayr et al. 2012). In the current study, removal rates of seeds did not differ among the seed treatments at any point of time, and both tagged seeds and untagged control seeds were removed from the dishes to a high degree (up to 100%) after being exposed for 25–35 days. For the flag-tagged seeds, flag color obviously did not affect removal rates. We can thus reject our third hypothesis. Contrary, Yi et al. (2008) observed significantly different temporal removal patterns of P. koraiensis seeds from experimental sites, with wire-plastic-tagged seeds being removed faster than wire-tin-tagged seeds. However, the removal rates of differently tagged seeds were quite similar at the end of the study (Yi et al. 2008).
Fixing of tags on seeds
For tying up wires or threads to seeds or for inserting magnets, small holes were drilled through the exocarp in many cases (e.g., Seiwa et al. 2002; Xiao et al. 2006; Yi et al. 2008). Although Cheng et al. (2005) assumed damages of cotyledons not affecting subsequent germination in case of still intact embryos, this method might cause piercing of cotyledons and ease infections by fungi thus influencing germination rates (Xiao et al. 2006) or trigger dehydration. Drilling a hole through a seed without damaging the embryo was successfully done for larger seeds like acorns [Q. serrate (Iida 2006), Q. variabilis (Xiao et al. 2006) or Q. liatungensis (Zhang et al. 2008)]. Smaller seeds like European beech (Fagus sylvatica) are more difficult to drill. Nevertheless, Wróbel and Zwolak (2013) used this method to tie tags to beechnuts. To avoid possibly negative effects of drilling on seed fates, we fixed wire (inclusive flat washers) and transmitters with solvent-free glue to the seeds, or used wire loop bonding. In our case, both methods allowed for successful application of tags on the seeds and for subsequent tracing of seeds. In the cases of tagging with wire threads and plastic tags we recommend wire loop bonding, as fixing the wire with flat washers and glue led to a loss of flat washers and subsequently the entire tagging in some cases. Wire loop bonding did not impede rodents´ foraging of seeds as the rate of consumed seeds with wire loops was higher compared to seeds equipped with radio-transmitters.
The effects of weights of tagged seeds on seed predators´ behavior
The tags used in this study increased seed weight by the factor 1.5 to 2.8 for the flag-tags and by the factor 4.8 to 7.3 for radio-transmitters. Past research has shown that seed weight and seed size affect seed predator’s behavior in terms of seed removal, caching histories, and consumption rates (Vander Wall 1990; Tamura and Hayashi 2008). Consequently, the added tags may have changed dispersers´ behavior in our study. However, for tracing of individual seed fates in densely covered forest ecosystems, trade-offs between weight-biased seed predator behavior (Xiao et al. 2013) and distance-biased recovery failures have to be found. Optical cues and salience of flag-tagged seeds might counterbalance discrimination capacities of small mammals, preferring heavier, sound seeds of obviously assumed higher nutritional value and dispersing them over larger distances (Hurly and Robertson 1987; Vander Wall 1995; Sone et al. 2002). Previous studies provided both evidence for longer dispersal distances of small mammalian zoochory for higher as well as for lower seed weights (see discussion in Tamura 2001).
It has been shown in several studies, that our focal dispersers (e.g., Apodemus. sp.) are handling distinctly heavier tree seeds than our radio-tagged seeds, reaching up to 40% to the body mass of mice (e.g., acorns of different Quercus or Juglans species, see Tamura 2001; Gómez et al. 2008; Tamura and Hayashi 2008). Accordingly, Soné and Kohno (1999) showed, that Apodemus speciosus Temminck specimen frequently transported acorns weighting more than 20% of their body mass. Muñoz and Bonal (2008) showed for A. sylvaticus that the probability of acorn (Q. ilex) removal substantially decreased at seed weights exceeding 60% of rodents´ biomass. We thus assume that our tagging methods–although increasing seed weights–provide meaningful data on the fate of dispersed beechnuts and on long-distance dispersal events.
Conclusions
Individual tracking of seed fates considerably improves the validity of models describing the probability distribution of dispersal kernels and estimating the contribution of zoochory to spatially and temporally differing forest regeneration dynamics. Balancing the trade off between weight-biased seed predator behavior (Xiao et al. 2013) and distance-biased recovery failures in densely covered forest ecosystems has been a challenge in such studies. In our test of (1) wire threads with plastic flags fixed with solvent-free glue, (2) wire threads with plastic flags twisted around a beechnut, and (3) radio-transmitters fixed with solvent-free glue, radio-tagged seeds exceeded weights of untagged beech seeds but weights were lower than maximum handling and dispersal weights of the focal dispersers (Apodemus sp.). Apart from weight aspects of seed-tag-assemblies, transport resistance of tagged seeds might also determine small mammals´ dispersal behavior. Seeds equipped with hard, flexible plastic tags might be abandoned due to their resistance to movement through dense vegetation (Yi et al. 2008). For our study, we assume that highly flexible plastic tags only yielded comparatively small transport resistance, as we did not observe abandoned flag-tagged seeds. Seed predators did not show differences in removing tagged or untagged seeds or differently tagged seeds at any point of time. Seeds with radio-transmitters experienced longer transport distances with a maximum of nearly 60 m. In one study area, higher portions of radio-tagged seeds could be recovered than for the flag-tagged seeds. Significantly more seeds tagged with radio-transmitters were cached than expected.
Thus, we recommend a combination of flag-tagging and radio-tracking methods (one tag per individual seed) under environmental conditions comparable to our study areas, in case that individual dispersal histories are of interest. We further recommend our method of fixing the tags to the beechnuts without drilling holes, whereby the method with wire loop bonding was more successful and did not increase the weight of the seeds that much.
References
Beck MJ, Vander Wall SB (2010) Seed dispersal by scatter-hoarding rodents in arid environments. J Ecol 98:1300–1309. https://doi.org/10.1111/j.1365-2745.2010.01716.x
Beckage B, Clark JS (2005) Does predation contribute to tree diversity? Oecologia 143:458–469. https://doi.org/10.1007/s00442-004-1815-9
Bonte D, Van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, Lehouck V, Matthysen E, Mustin K, Saastamoinen M, Schtickzelle N, Stevens VM, Vandewoestijne S, Baguette M, Barton K, Benton TG, Chaput-Bardy A, Clobert J, Dytham C, Hovestadt T, Meier CM, Palmer SCF, Turlure C, Travis JMJ (2012) Costs of dispersal. Biol Rev 87:290–312. https://doi.org/10.1111/j.1469-185X.2011.00201.x
Brenner H, Drozdowski I, Mrkvicka A (2015) Wälder im Wienerwald. Biosphärenpark Wienerwald Management GmbH. https://www.bpww.at/sites/default/files/download_files/Waldbuechlein_Homepage_SMALL.pdf. Accessed 10 December 2017
Burschel P (1966) Untersuchungen in Buchenmastjahren. Forstw Cbl 85:204–219. https://doi.org/10.1007/BF01829024
Carlo TA, García D, Martínez D, Gleditsch JM, Morales JM (2013) Where do seeds go when they go far? Distance and directionality of avian seed dispersal in heterogeneous landscapes. Ecology 94:301–307. https://doi.org/10.1890/12-0913.1
Cheng J, Xiao Z, Zhang Z (2005) Seed consumption and caching on seeds of three sympatric tree species by four sympatric rodent species in a subtropical forest, China. For Ecol Manag 216:331–341. https://doi.org/10.1016/j.foreco.2005.05.045
Cilles SE, Coy G, Stieha CR, Cox JJ, Crowley PH, Maehr DS (2016) A comparison of seed predation, seed dispersal, and seedling herbivory in oak and hickory: species with contrasting regenerating abilities in a bluegrass savanna—woodland habitat. Northeast Nat 23:466–481. https://doi.org/10.1656/045.023.0404
Clarkson K, Eden SF, Sutherland WJ, Houston AI (1986) Density dependence and magpie food hoarding. J Anim Ecol 55:111–121. https://doi.org/10.2307/4696
Cody ML, Smallwood JA (1996) Long-term studies of vertebrate communities. Academic Press, Cambridge
Corbet GB, Ovenden D (1980) The mammals of Britain and Europe. HarperCollins Publisher, Glasgow
Cousens R, Dytham C, Law R (2008) Dispersal in plants: a population perspective. Oxford University Press, Oxford
Ellmauer T (2011) Die Blumen des Wildnisgebietes Dürrenstein. Schutzgebietsverwaltung Wildnisgebiet Dürrenstein, Scheibbs
Forget P-M, Wenny D (2005) How to elucidate seed fate? A review of methods used to study seed removal and secondary seed dispersal. In: Forget P-M, Lambert JE, Hulme PE, Vander Wall SB (eds) Seed fate: predation, dispersal and seedling establishment. CABI Publishing, Wallingford, pp 379–393
Gómez JM, Puerta-Piñero C, Schupp EW (2008) Effectiveness of rodents as local seed dispersers of Holm oaks. Oecologia 155:529–537. https://doi.org/10.1007/s00442-007-0928-3
Görner M, Hackethal H (1988) Säugetiere Europas. dtv Deutscher Taschenbuchverlag, Munich
Guo K, Li R, Werger M (2001) Effect of acorn burying depth on germination, seedling emergence and development of Quercus aliena var. acuteserrata. Acta Bot Sin 43:974–978
Haas JP, Heske EJ (2005) Experimental study of the effects of mammalian acorn predators on red oak acorn survival and germination. J Mammal 86:1015–1021. https://doi.org/10.1644/1545-1542(2005)86[1015:ESOTEO]2.0.CO;2
Hasenkamp N, Ziegenhagen B, Mengel C, Schulze L, Schmitt H-P, Liepelt S (2011) Towards a DNA marker assisted seed source identification: a pilot study in European beech (Fagus sylvatica L.). Eur J For Res 130:513–519. https://doi.org/10.1007/s10342-010-0439-3
Hirsch BT, Kays R, Jansen PA (2012) A telemetric thread tag for tracking seed dispersal by scatter-hoarding rodents. Plant Ecol 213:933–943. https://doi.org/10.1007/s11258-012-0054-0
Hurly TA, Robertson RJ (1987) Scatterhoarding by territorial red squirrels: a test of the optimal density model. Can J Zool 65:1247–1252. https://doi.org/10.1139/z87-194
Iida S (2006) Dispersal patterns of Quercus serrata acorns by wood mice in and around canopy gaps in a temperate forest. For Ecol Manag 227:71–78. https://doi.org/10.1016/j.foreco.2006.02.010
Jansen PA, Bartholomeus M, Bongers F, Elzinga JA, den Ouden J, Van Wieren SE (2002) The role of seed size in dispersal by a scatter-hoarding rodent. In: Levey DJ, Silva WR, Galetti M (eds) Seed dispersal and frugivory: ecology, evolution, and conservation. CABI Publishing, São Pedro, pp 209–225
Jensen TS (1985) Seed-seed predator interactions of European beech, Fagus silvatica and forest rodents, Clethrionomys glareolus and Apodemus flavicollis. Oikos 44:149. https://doi.org/10.2307/3544056
Kempter I, Nopp-Mayr U (2013) Langzeit-Monitoring von Kleinsäugern im Wildnisgebiet Dürrenstein. Silva Fera 2:94–99
Kitamura S, Yumoto T, Poonswad P, Suzuki S, Wohandee P (2008) Rare seed-predating mammals determine seed fate of Canarium euphyllum, a large-seeded tree species in a moist evergreen forest, Thailand. Ecol Res 23:169–177. https://doi.org/10.1007/s11284-007-0350-7
Lemke A, Von der Lippe M, Kowarik I (2009) New opportunities for an old method: using fluorescent colours to measure seed dispersal. J Appl Ecol 46:1122–1128. https://doi.org/10.1111/j.1365-2664.2009.01691.x
Li H-J, Zhang Z-B (2003) Effect of rodents on acorn dispersal and survival of the Liaodong oak (Quercus liaotungensis Koidz.). For Ecol Manag 176:387–396. https://doi.org/10.1016/S0378-1127(02)00286-4
Lichti NI, Steele MA, Swihart RK (2017) Seed fate and decision-making processes in scatter-hoarding rodents. Biol Rev 92:474–504. https://doi.org/10.1111/brv.12240
Moore JE, McEuen AB, Swihart RK, Contreras TA, Steele MA (2007) Determinants of seed removal distance by scatter-hoarding rodents in deciduous forests. Ecology 88:2529–2540. https://doi.org/10.1890/07-0247.1
Morán-López T, Fernández M, Alonso CL, Flores-Rentería D, Valladares F, Díaz M (2015) Effects of forest fragmentation on the oak-rodent mutualism. Oikos 124:1482–1491. https://doi.org/10.1111/oik.02061
Muñoz A, Bonal R (2008) Are you strong enough to carry that seed? Seed size/body size ratios influence seed choices by rodents. Anim Behav 76:709–715. https://doi.org/10.1016/j.anbehav.2008.03.017
Muñoz A, Bonal R (2011) Linking seed dispersal to cache protection strategies: seed dispersal and caching strategies. J Ecol 99:1016–1025. https://doi.org/10.1111/j.1365-2745.2011.01818.x
Nathan R, Perry G, Cronin JT, Strand AE, Cain ML (2003) Methods for estimating long-distance dispersal. Oikos 103:261–273. https://doi.org/10.1034/j.1600-0706.2003.12146.x
Nathan R, Klein E, Robledo-Arnuncio JJ, Revilla E (2012) Dispersal kernels: review. In: Clobert J, Baguette M, Benton TG, Bullock JM (eds) Dispersal ecology and evolution. Oxford University, Oxford, pp 187–210
Niethammer J, Krapp F (1978) Handbuch der Säugetiere Europas. Aula-Verlag, Wiesbaden
Niethammer J, Krapp F (1982) Handbuch der Säugetiere Europas. Aula-Verlag, Wiesbaden
Nopp-Mayr U, Kempter I, Muralt G, Gratzer G (2012) Seed survival on experimental dishes in a central European old-growth mixed-species forest—effects of predator guilds, tree masting and small mammal population dynamics. Oikos 121:337–346. https://doi.org/10.1111/j.1600-0706.2011.19099.x
Nopp-Mayr U, Kempter I, Muralt G, Gratzer G (2015) Herbivory on young tree seedlings in old-growth and managed mountain forests. Ecol Res 30:479–491. https://doi.org/10.1007/s11284-015-1247-5
Pons J, Pausas JG (2007) Acorn dispersal estimated by radio-tracking. Oecologia 153:903–911. https://doi.org/10.1007/s00442-007-0788-x
Rohmeder E (1972) Das Saatgut in der Forstwirtschaft. Paul Parey, Hamburg
Saastamoinen M, Bocedi G, Cote J, Legrand D, Giullaume F, Wheat CW, Fronhofer EA, Garcia C, Henry R, Husby A, Baguette M, Bonte D, Coulon A, Kokko H, Matthysen E, Niitepõld K, Nonaka E, Stevens VM, Travis JMJ, Donohue K, Bullock JM, del Mar Delgado M (2018) Genetics of dispersal. Biol Rev 93:574–599. https://doi.org/10.1111/brv.12356
Schnurr JL, Ostfeld RS, Canham CD (2002) Direct and indirect effects of masting on rodent populations and tree seed survival. Oikos 96:402–410. https://doi.org/10.1034/j.1600-0706.2002.960302.x
Schnurr JL, Canham CD, Ostfeld RS, Inouye RS (2004) Neighborhood analyses of small-mammal dynamics: impacts on seed predation and seedling establishment. Ecology 85:741–755. https://doi.org/10.1890/02-0644
Seiwa K, Watanabe A, Irie K, Kanno H, Saitoh T, Akasaka S (2002) Impact of site-induced mouse caching and transport behaviour on regeneration in Castanea crenata. J Veg Sci 13:517–526. https://doi.org/10.1658/1100-9233(2002)013[0517:IOSIMC]2.0.CO;2
Soné K, Kohno A (1999) Acorn hoarding by the field mouse, Apodemus speciosus Temminck (Rodentia: Muridae). J For Res 4:167–175. https://doi.org/10.1007/BF02762243
Sone K, Hiroi S, Nagahama D, Ohkubo C, Nakano E, Murao S-I, Hata K (2002) Hoarding of acorns by granivorous mice and its role in the population processes of Pasania edulis (Makino) Makino. Ecol Res 17:553–564. https://doi.org/10.1046/j.1440-1703.2002.00514.x
Stapanian MA, Smith CC (1978) A model for seed scatterhoarding: coevolution of fox squirrels and black walnuts. Ecology 59:884–896
Stradiotto A, Cagnacci F, Delahay R, Tioli S, Nieder L, Rizzoli A (2009) Spatial organization of the yellow-necked mouse: effects of density and resource availability. J Mamm 90:704–714
Sunyer P, Boixadera E, Muñoz A, Bonal R, Espelta JM (2015) The interplay among acorn abundance and rodent behavior drives the spatial pattern of seedling recruitment in mature mediterranean oak forests. PLoS One 10:e0129844. https://doi.org/10.1371/journal.pone.0129844
Suselbeek L, Jansen PA, Prins HHT, Steele MA (2013) Tracking rodent-dispersed large seeds with Passive Integrated Transponder (PIT) tags. Methods Ecol Evol 4:513–519. https://doi.org/10.1111/2041-210X.12027
Tamura N (2001) Walnut hoarding by the Japanese wood mouse, Apodemus speciosus Temminck. J For Res 6:187–190. https://doi.org/10.1007/BF02767091
Tamura N, Hayashi F (2008) Geographic variation in walnut seed size correlates with hoarding behaviour of two rodent species. Ecol Res 23:607–614. https://doi.org/10.1007/s11284-007-0414-8
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 11 October 2017
Vander Wall SB (1990) Food hoarding in animals. The University of Chicago, Chicago
Vander Wall SB (1995) The effects of seed value on the caching behavior of yellow pine chipmunks. Oikos 74:533–537. https://doi.org/10.2307/3545999
Vander Wall SB (2010) How plants manipulate the scatter-hoarding behaviour of seed-dispersing animals. Philos Trans R Soc B Biol Sci 365:989–997. https://doi.org/10.1098/rstb.2009.0205
Vander Wall SB, Jenkins SH (2003) Reciprocal pilferage and the evolution of food-hoarding behavior. Behav Ecol 14:656–667. https://doi.org/10.1093/beheco/arg064
Wang B, Ives AR (2017) Tree-to-tree variation in seed size and its consequences for seed dispersal versus predation by rodents. Oecologia 183:751–762. https://doi.org/10.1007/s00442-016-3793-0
Wang B, Wang G, Chen J (2012) Scatter-hoarding rodents use different foraging strategies for seeds from different plant species. Plant Ecol 213:1329–1336. https://doi.org/10.1007/s11258-012-0091-8
Watts CHS (1970) Long distance movement of bank voles and wood mice. J Zool 161:247–256. https://doi.org/10.1111/j.1469-7998.1970.tb02039.x
Wenny DG (2000) Seed dispersal, seed predation, and seedling recruitment of a neotropical montane tree. Ecol Monogr 70:331–351. https://doi.org/10.1890/0012-9615(2000)070[0331:SDSPAS]2.0.CO;2
Wróbel A, Zwolak R (2013) The choice of seed tracking method influenced fate of beech seeds dispersed by rodents. Plant Ecol 214:471–475. https://doi.org/10.1007/s11258-013-0183-0
Xiao Z, Zhang Z, Wang Y (2005) Effects of seed size on dispersal distance in five rodent-dispersed fagaceous species. Acta Oecol 28:221–229. https://doi.org/10.1016/j.actao.2005.04.006
Xiao Z, Jansen PA, Zhang Z (2006) Using seed-tagging methods for assessing post-dispersal seed fate in rodent-dispersed trees. For Ecol Manag 223:18–23. https://doi.org/10.1016/j.foreco.2005.10.054
Xiao Z, Gao X, Zhang Z (2013) The combined effects of seed perishability and seed size on hoarding decisions by Pére David’s rock squirrels. Behav Ecol Sociobiol 67:1067–1075. https://doi.org/10.1007/s00265-013-1531-8
Yi X, Wang Z (2015) Tracking animal-mediated seedling establishment from dispersed acorns with the aid of the attached cotyledons. Mammal Res 60:149–154. https://doi.org/10.1007/s13364-015-0214-4
Yi X, Xiao Z, Zhang Z (2008) Seed dispersal of Korean pine Pinus koraiensis labeled by two different tags in a northern temperate forest, northeast China. Ecol Res 23:379–384. https://doi.org/10.1007/s11284-007-0392-x
Yi X, Liu G, Zhang M, Dong Z, Yang Y (2014) A new approach for tracking seed dispersal of large plants: soaking seeds with 15 N-urea. Ann For Sci 71:43–49. https://doi.org/10.1007/s13595-013-0331-7
Zar JH (2010) Biostatistical analysis, 5th edn. Prentice Hall, New Jersey
Zhang H, Chen Y, Zhang Z (2008) Differences of dispersal fitness of large and small acorns of Liaodong oak (Quercus liaotungensis) before and after seed caching by small rodents in a warm temperate forest, China. For Ecol Manag 255:1243–1250. https://doi.org/10.1016/j.foreco.2007.10.028
Zöfel P (1992) Statistik in der Praxis. UTB, Stuttgart
Zukrigl K, Eckhart G, Nather J (1963) Standortskundliche und waldbauliche Untersuchungen in Urwaldresten der niederösterreichischen Kalkalpen. Forstliche Bundesversuchsanstalt, Mariabrunn
Acknowledgements
We thank Dr. Christoph Leditznig (Wilderness Area Dürrenstein) for financial support of small mammal live trapping and DI Johannes Doppler (Forest Administration of Langau) as well as the Biosphere Reserve Wienerwald Management GmbH for access to the study area. Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Welfare of animals
Experiments were done in compliance with the Austrian laws.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kempter, I., Nopp-Mayr, U., Hausleithner, C. et al. Tricky to track: comparing different tagging methods for tracing beechnut dispersal by small mammals. Ecol Res 33, 1219–1231 (2018). https://doi.org/10.1007/s11284-018-1640-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-018-1640-y