Abstract
Objective
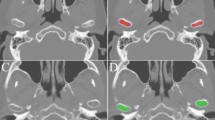
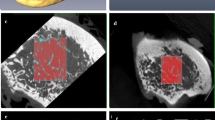
Texture analysis is an image processing method that aims to assess the distribution of gray-level intensity and spatial organization of the pixels in the image. The purpose of this study was to investigate whether the texture analysis applied to cone beam computed tomography (CBCT) images could detect variation in the condyle trabecular bone of individuals from different age groups and genders.
Methods
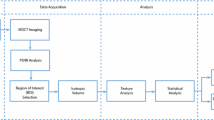
The sample consisted of imaging exams from 63 individuals divided into three groups according to age groups of 03–13, 14–24 and 25–34. For texture analysis, the MaZda® software was used to extract the following parameters: second angular momentum, contrast, correlation, sum of squares, inverse difference moment, sum entropy and entropy. Statistical analysis was performed using Mann–Whitney test for gender and Kruskal–Wallis test for age (P = 5%).
Results
No statistically significant differences were found between age groups for any of the parameters. Males had lower values for the parameter correlation than those of females (P < 0.05).
Conclusion
Texture analysis proved to be useful to discriminate mandibular condyle trabecular bone between genders.

Similar content being viewed by others
References
de Oliveira MS, Balthazar ML, D’Abreu A, et al. MR imaging texture analysis of the corpus callosum and thalamus in amnestic mild cognitive impairment and mild Alzheimer disease. AJNR Am J Neuroradiol. 2011;32(1):60–6. https://doi.org/10.3174/ajnr.A2232.
Caruso D, Zerunian M, Ciolina M, et al. Haralick’s texture features for the prediction of response to therapy in colorectal cancer: a preliminary study. Radiol Med. 2018;123(3):161–7. https://doi.org/10.1007/s11547-017-0833-8.
Maas M, Lambregts DM, Lahaye MJ, et al. T-staging of rectal cancer: accuracy of 3.0 Tesla MRI compared with 1.5 Tesla. Abdom Imaging. 2012;37(3):475–81. https://doi.org/10.1007/s00261-011-9770-5.
De Rosa CS, Bergamini ML, Palmieri M, et al. Differentiation of periapical granuloma from radicular cyst using cone beam computed tomography images texture analysis. Heliyon. 2020;6(10): e05194. https://doi.org/10.1016/j.heliyon.2020.e05194.
de Albuquerque M, Anjos LG, Tavares M, de Andrade H, et al. MRI texture analysis reveals deep gray nuclei damage in amyotrophic lateral sclerosis. J Neuroimaging. 2016;26(2):201–6. https://doi.org/10.1111/jon.12262.
Bonilha L, Kobayashi E, Castellano G, et al. Texture analysis of hippocampal sclerosis. Epilepsia. 2003;44(12):1546–50.
Raja JV, Khan M, Ramachandra VK, et al. Texture analysis of CT images in the characterization of oral cancers involving buccal mucosa. Dentomaxillofac Radiol. 2012;41(6):475–80. https://doi.org/10.1259/dmfr/83345935.
Ramkumar S, Ranjbar S, Ning S, et al. MRI-based texture analysis to differentiate sinonasal squamous cell carcinoma from inverted papilloma. AJNR Am J Neuroradiol. 2017;38(5):1019–25. https://doi.org/10.3174/ajnr.A5106.
MacKay JW, Kapoor G, Driban JB, et al. Association of subchondral bone texture on magnetic resonance imaging with radiographic knee osteoarthritis progression: data from the Osteoarthritis Initiative Bone Ancillary Study. Eur Radiol. 2018;28(11):4687–95. https://doi.org/10.1007/s00330-018-5444-9.
Fruehwald-Pallamar J, Czerny C, Holzer-Fruehwald L, et al. Texture-based and diffusion-weighted discrimination of parotid gland lesions on MR images at 3.0 Tesla. NMR Biomed. 2013;26(11):1372–9. https://doi.org/10.1002/nbm.2962.
Miles KA, Ganeshan B, Hayball MP. CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging. 2013;13(3):400–6. https://doi.org/10.1102/1470-7330.2013.9045.
Costa ALF, de Souza CB, Fardim KAC, et al. Texture analysis of cone beam computed tomography images reveals dental implant stability. Int J Oral Maxillofac Surg. 2021. https://doi.org/10.1016/j.ijom.2021.04.009.
Castellano G, Bonilha L, Li LM, et al. Texture analysis of medical images. Clin Radiol. 2004;59(12):1061–9. https://doi.org/10.1016/j.crad.2004.07.008.
De Cecco CN, Ganeshan B, Ciolina M, et al. Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol. 2015;50(4):239–45. https://doi.org/10.1097/RLI.0000000000000116.
Bianchi J, Goncalves JR, Ruellas ACO, et al. Software comparison to analyze bone radiomics from high resolution CBCT scans of mandibular condyles. Dentomaxillofac Radiol. 2019;48(6):20190049. https://doi.org/10.1259/dmfr.20190049.
Zaccagna F, Ganeshan B, Arca M, et al. CT texture-based radiomics analysis of carotid arteries identifies vulnerable patients: a preliminary outcome study. Neuroradiology. 2021;63(7):1043–52. https://doi.org/10.1007/s00234-020-02628-0.
Dieckmeyer M, Junker D, Ruschke S, et al. Vertebral bone marrow heterogeneity using texture analysis of chemical shift encoding-based mri: variations in age, sex, and anatomical location. Front Endocrinol (Lausanne). 2020;11: 555931. https://doi.org/10.3389/fendo.2020.555931.
Ling H, Yang X, Li P, et al. Cross gender-age trabecular texture analysis in cone beam CT. Dentomaxillofac Radiol. 2014;43(4):20130324. https://doi.org/10.1259/dmfr.20130324.
Vujasinovic T, Pribic J, Kanjer K, et al. Gray-level co-occurrence matrix texture analysis of breast tumor images in prognosis of distant metastasis risk. Microsc Microanal. 2015;21(3):646–54. https://doi.org/10.1017/S1431927615000379.
Haralick RS. K; Dinstein I textural features for image classification. IEEE Trans Syst Man Cybern. 1973;3:610–21.
Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–9. https://doi.org/10.2215/CJN.04151206.
Amin S, Khosla S. Sex- and age-related differences in bone microarchitecture in men relative to women assessed by high-resolution peripheral quantitative computed tomography. J Osteoporos. 2012;2012: 129760. https://doi.org/10.1155/2012/129760.
Dalzell N, Kaptoge S, Morris N, et al. Bone micro-architecture and determinants of strength in the radius and tibia: age-related changes in a population-based study of normal adults measured with high-resolution pQCT. Osteoporos Int. 2009;20(10):1683–94. https://doi.org/10.1007/s00198-008-0833-6.
Widmalm SE, Westesson PL, Kim IK, et al. Temporomandibular joint pathosis related to sex, age, and dentition in autopsy material. Oral Surg Oral Med Oral Pathol. 1994;78(4):416–25. https://doi.org/10.1016/0030-4220(94)90031-0.
Jiao K, Dai J, Wang MQ, et al. Age- and sex-related changes of mandibular condylar cartilage and subchondral bone: a histomorphometric and micro-CT study in rats. Arch Oral Biol. 2010;55(2):155–63. https://doi.org/10.1016/j.archoralbio.2009.11.012.
Mulder L, Koolstra JH, Van Eijden TM. Accuracy of MicroCT in the quantitative determination of the degree and distribution of mineralization in developing bone. Acta Radiol. 2006;47(8):882–3. https://doi.org/10.1080/02841850600854944.
Parfitt AM. Age-related structural changes in trabecular and cortical bone: cellular mechanisms and biomechanical consequences. Calcif Tissue Int. 1984;36(Suppl 1):S123–8. https://doi.org/10.1007/BF02406145.
Goncalves BC, de Araujo EC, Nussi AD, et al. Texture analysis of cone-beam computed tomography images assists the detection of furcal lesion. J Periodontol. 2020. https://doi.org/10.1002/JPER.19-0477.
Haralick RM. Texture features for image classification. IEEE Trans Syst Man Cybern. 1973;SMC-3(6):610–621.
Lubner MG, Smith AD, Sandrasegaran K, et al. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. Radiographics. 2017;37(5):1483–503. https://doi.org/10.1148/rg.2017170056.
Ranjanomennahary P, Ghalila SS, Malouche D, et al. Comparison of radiograph-based texture analysis and bone mineral density with three-dimensional microarchitecture of trabecular bone. Med Phys. 2011;38(1):420–8. https://doi.org/10.1118/1.3528125.
Ganeshan B, Skogen K, Pressney I, et al. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol. 2012;67(2):157–64. https://doi.org/10.1016/j.crad.2011.08.012.
Mungai F, Verrone GB, Pietragalla M, et al. CT assessment of tumor heterogeneity and the potential for the prediction of human papillomavirus status in oropharyngeal squamous cell carcinoma. Radiol Med. 2019. https://doi.org/10.1007/s11547-019-01028-6.
Gong H, Zhu D, Gao J, et al. An adaptation model for trabecular bone at different mechanical levels. Biomed Eng Online. 2010;2(9):32. https://doi.org/10.1186/1475-925X-9-32.
Cosgunarslan A, SoydanCabuk D, Canger EM. Effect of total edentulism on the internal bone structure of mandibular condyle: a preliminary study. Oral Radiol. 2021;37(2):268–75. https://doi.org/10.1007/s11282-020-00444-z.
Gulec M, Tassoker M, Ozcan S, et al. Evaluation of the mandibular trabecular bone in patients with bruxism using fractal analysis. Oral Radiol. 2021;37(1):36–45. https://doi.org/10.1007/s11282-020-00422-5.
Harrison LC, Nikander R, Sikio M, et al. MRI texture analysis of femoral neck: detection of exercise load-associated differences in trabecular bone. J Magn Reson Imaging. 2011;34(6):1359–66. https://doi.org/10.1002/jmri.22751.
Maciel JG, Araujo IM, Trazzi LC, et al. Association of bone mineral density with bone texture attributes extracted using routine magnetic resonance imaging. Clinics (Sao Paulo). 2020;75: e1766. https://doi.org/10.6061/clinics/2020/e1766.
Funding
This study was supported by FAPESP (São Paulo Research Foundation) grants: 2017/09550-4 and 2019/00495-6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors of this work declare no conflict of interest.
Ethics approval
The study was approved by the Institutional Review Board of USP, according to protocol number 31575020.8.0000.0075. All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Informed consent
Written informed consent was obtained from the guardian of each participant, after informed about the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nussi, A.D., de Castro Lopes, S.L.P., De Rosa, C.S. et al. In vivo study of cone beam computed tomography texture analysis of mandibular condyle and its correlation with gender and age. Oral Radiol 39, 191–197 (2023). https://doi.org/10.1007/s11282-022-00620-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11282-022-00620-3