Abstract
Objectives
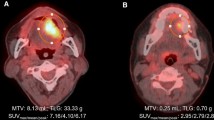
This study aimed to create a predictive model for cervical lymph node metastasis (CLNM) in patients with tongue squamous cell carcinoma (SCC) based on radiomics features detected by [18F]-fluoro-2-deoxyglucose (18F-FDG) positron emission tomography (PET).
Methods
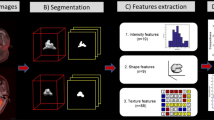
A total of 40 patients with tongue SCC who underwent 18F-FDG PET imaging during their first medical examination were enrolled. During the follow-up period (mean 28 months), 20 patients had CLNM, including six with late CLNM, whereas the remaining 20 patients did not have CLNM. Radiomics features were extracted from 18F-FDG PET images of all patients irrespective of metal artifact, and clinicopathological factors were obtained from the medical records. Late CLNM was defined as the CLNM that occurred after major treatment. The least absolute shrinkage and selection operator (LASSO) model was used for radiomics feature selection and sequential data fitting. The receiver operating characteristic curve analysis was used to assess the predictive performance of the 18F-FDG PET-based model and clinicopathological factors model (CFM) for CLNM.
Results
Six radiomics features were selected from LASSO analysis. The average values of the area under the curve (AUC), accuracy, sensitivity, and specificity of radiomics analysis for predicting CLNM from 18F-FDG PET images were 0.79, 0.68, 0.65, and 0.70, respectively. In contrast, those of the CFM were 0.54, 0.60, 0.60, and 0.60, respectively. The 18F-FDG PET-based model showed significantly higher AUC than that of the CFM.
Conclusions
The 18F-FDG PET-based model has better potential for diagnosing CLNM and predicting late CLNM in patients with tongue SCC than the CFM.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, TK, upon reasonable request.
Change history
11 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11282-022-00631-0
References
Castelijns JA, van den Brekel MW. Detection of lymph node metastases in the neck: radiologic criteria. AJNR Am J Neuroradiol. 2001;22:3–4. https://doi.org/10.1148/radiology.192.3.8058923.
Eida S, Sumi M, Yonetsu K, Kimura Y, Nakamura T. Combination of helical CT and Doppler sonography in the follow-up of patients with clinical N0 stage neck disease and oral cancer. AJNR Am J Neuroradiol. 2003;24:312–8.
Schöder H, Carlson DL, Kraus DH, Stambuk HE, Gönen M, Erdi YE, et al. 18F-FDG PET/CT for detecting nodal metastases in patients with oral cancer staged N0 by clinical examination and CT/MRI. J Nucl Med. 2006;47:755–62.
Pandeshwar P, Jayanthi K, Raghuram P. Pre-operative contrast enhanced computer tomographic evaluation of cervical nodal metastatic disease in oral squamous cell carcinoma. Indian J Cancer. 2013;50:310–5. https://doi.org/10.4103/0019-509X.123605.
Pfister DG, Ang K, Brizel DM, Burtness BA, Cmelak AJ, Colevas AD, et al. Head and Neck Cancers, version 3.2021, NCCN clinical practice guidelines in oncology. Accessed 20 Sep 2021. http://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437;9:596–650;9:596–650. https://doi.org/10.6004/jnccn.2011.0053
D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015;373:521–9. https://doi.org/10.1056/NEJMoa1506007.
Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head Neck. 1997;19:583–8. https://doi.org/10.1002/(SICI)1097-0347(199710)19:7%3c583::AID-HED4%3e3.0.CO;2-3.
Lim YC, Lee JS, Koo BS, Kim SH, Kim YH, Choi EC. Treatment of contralateral N0 neck in early squamous cell carcinoma of the oral tongue: elective neck dissection versus observation. Laryngoscope. 2006;116:461–5. https://doi.org/10.1097/01.mlg.0000195366.91395.9b.
Kelner N, Vartanian JG, Pinto CA, Coutinho-Camillo CM, Kowalski LP. Does elective neck dissection in T1/T2 carcinoma of the oral tongue and floor of the mouth influence recurrence and survival rates? Br J Oral Maxillofac Surg. 2014;52:590–7. https://doi.org/10.1016/j.bjoms.2014.03.020.
Zhong Y, Yuan M, Zhang T, Zhang YD, Li H, Yu TF. Radiomics approach to prediction of occult mediastinal lymph node metastasis of lung adenocarcinoma. AJR Am J Roentgenol. 2018;211:109–13. https://doi.org/10.2214/AJR.17.19074.
Cui X, Wang N, Zhao Y, Chen S, Li S, Xu M, et al. Preoperative prediction of axillary lymph node metastasis in breast cancer using radiomics features of DCE-MRI. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-38502-0.
Japanese Society for Head and Neck cancer guidelines for the treatment of oral cancer. Accessed 30 Jun 2021. http://www.jsco-cpg.jp/headandneck-cancer/algo/#III-B-1
Japanese Society of Oral Oncology guidelines for the treatment of oral cancer. Accessed 30 Jun 2021. https://www.jstage.jst.go.jp/article/jsot1989/19/3/19_3_139/_pdf/-char/ja
Yamamoto E, Miyakawa A, Kohama G. Mode of invasion and lymph node metastasis in squamous cell carcinoma of the oral cavity. Head Neck Surg. 1984;6:938–47. https://doi.org/10.1002/hed.2890060508.
Vallières M, Freeman CR, Skamene SR, El Naqa I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol. 2015;60:5471–96. https://doi.org/10.1088/0031-9155/60/14/5471.
Haga A, Takahashi W, Aoki S, Nawa K, Yamashita H, Abe O, et al. Classification of early stage non-small cell lung cancers on computed tomographic images into histological types using radiomic features: interobserver delineation variability analysis. Radiol Phys Technol. 2018;11:27–35. https://doi.org/10.1007/s12194-017-0433-2.
Di Martino E, Nowak B, Hassan HA, Hausmann R, Adam G, Buell U, et al. Diagnosis and staging of head and neck cancer: a comparison of modern imaging modalities (positron emission tomography, computed tomography, color-coded duplex sonography) with panendoscopic and histopathologic findings. Arch Otolaryngol Head Neck Surg. 2000;126:1457–61. https://doi.org/10.1001/archotol.126.12.1457.
Ahn PH, Garg MK. Positron emission tomography/computed tomography for target delineation in head and neck cancers. Semin Nucl Med. 2008;38:141–8. https://doi.org/10.1053/j.semnuclmed.2007.11.002.
Houweling AC, Wolf AL, Vogel WV, Hamming-Vrieze O, van Vliet-Vroegindeweij CV, van de Kamer JB, et al. FDG-PET and diffusion-weighted MRI in head-and-neck cancer patients: implications for dose painting. Radiother Oncol. 2013;106:250–4. https://doi.org/10.1016/j.radonc.2013.01.003.
Yan O, Wang H, Han Y, Fu S, Chen Y, Liu F. Prognostic relevance of 18F-FDG-PET/CT-guided target volume delineation in loco-regionally advanced nasopharyngeal carcinomas: a comparative study. Front Oncol. 2021;11: 709622. https://doi.org/10.3389/fonc.2021.709622.
Lee SJ, Choi JY, Lee HJ, Baek CH, Son YI, Hyun SH, et al. Prognostic value of volume-based 18F-fluorodeoxyglucose PET/CT parameters in patients with clinically node-negative oral tongue squamous cell carcinoma. Korean J Radiol. 2012;13:752–9. https://doi.org/10.3348/kjr.2012.13.6.752.
Thomas TO, Refaat T, Choi M, Bacchus I, Sachdev S, Rademaker AW, et al. Brachial plexus dose tolerance in head and neck cancer patients treated with sequential intensity modulated radiation therapy. Radiat Oncol. 2015;10:94. https://doi.org/10.1186/s13014-015-0409-5.
Merlotti A, Alterio D, Vigna-Taglianti RV, Muraglia A, Lastrucci L, Manzo R, et al. Technical guidelines for head and neck cancer IMRT on behalf of the Italian association of radiation oncology - head and neck working group. Radiat Oncol. 2014;9:264. https://doi.org/10.1186/s13014-014-0264-9.
Zhou Z, Chen L, Sher D, Zhang Q, Shah J, Pham NL, et al. Predicting lymph node metastasis in head and neck cancer by combining many-objective radiomics and 3-dimensional convolutional neural network through evidential reasoning. Annu Int Conf IEEE Eng Med Biol Soc. 2018. https://doi.org/10.1109/EMBC.2018.8513070.
Haider SP, Zeevi T, Baumeister P, Reichel C, Sharaf K, Forghani R, et al. Potential added value of PET/CT radiomics for survival prognostication beyond AJCC 8th edition staging in oropharyngeal squamous cell carcinoma. Cancers (Basel). 2020;12:1778. https://doi.org/10.3390/cancers12071778.
Martens RM, Koopman T, Noij DP, Pfaehler E, Übelhör C, Sharma S, et al. Predictive value of quantitative 18F-FDG-PET radiomics analysis in patients with head and neck squamous cell carcinoma. EJNMMI Res. 2020;10:102. https://doi.org/10.1186/s13550-020-00686-2.
Chen L, Zhou Z, Sher D, Zhang Q, Shah J, Pham NL, et al. Combining many-objective radiomics and 3D convolutional neural network through evidential reasoning to predict lymph node metastasis in head and neck cancer. Phys Med Biol. 2019;64: 075011. https://doi.org/10.1088/1361-6560/ab083a.
Zhai TT, Langendijk JA, van Dijk LV, Halmos GB, Witjes MJH, Oosting SF, et al. The prognostic value of CT-based image-biomarkers for head and neck cancer patients treated with definitive (chemo-)radiation. Oral Oncol. 2019;95:178–86. https://doi.org/10.1016/j.oraloncology.2019.06.020.
Diamant A, Chatterjee A, Vallières M, Shenouda G, Seuntjens J. Deep learning in head & neck cancer outcome prediction. Sci Rep. 2019;9:2764. https://doi.org/10.1038/s41598-019-39206-1.
Romeo V, Cuocolo R, Ricciardi C, Ugga L, Cocozza S, Verde F, et al. Prediction of tumor grade and nodal status in oropharyngeal and oral cavity squamous-cell carcinoma using a radiomic approach. Anticancer Res. 2020;40:271–80.
Miki K, Mori S, Hasegawa A, Naganawa K, Koto M. Single-energy metal artefact reduction with CT for carbon-ion radiation therapy treatment planning. Br J Radiol. 2016;89:20150988. https://doi.org/10.1259/bjr.20150988.
Arena L, Morehouse HT, Safir J. MR imaging artifacts that simulate disease: how to recognize and eliminate them. Radiographics. 1995;15:1373–94. https://doi.org/10.1148/radiographics.15.6.8577963.
Kaneda T, Minami M, Curtin HD, Utsunomiya T, Shirouzu I, Yamashiro M, et al. Dental bur fragments causing metal artifacts on MR images. AJNR Am J Neuroradiol. 1998;19:317–9.
Huang SH, Hwang D, Lockwood G, Goldstein DP, O’Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009;115:1489–97. https://doi.org/10.1002/cncr.24161.
Bur AM, Holcomb A, Goodwin S, Woodroof J, Karadaghy O, Shnayder Y, et al. Machine learning to predict occult nodal metastasis in early oral squamous cell carcinoma. Oral Oncol. 2019;92:20–5. https://doi.org/10.1016/j.oraloncology.2019.03.011.
Shaha AR, Spiro RH, Shah JP, Strong EW. Squamous carcinoma of the floor of the mouth. Am J Surg. 1984;148:455–9. https://doi.org/10.1016/0002-9610(84)90369-6.
Spiro RH, Huvos AG, Wong GY, Spiro JD, Gnecco CA, Strong EW. Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg. 1986;152:345–50. https://doi.org/10.1016/0002-9610(86)90302-8.
Rodolico V, Barresi E, Di Lorenzo R, Leonardi V, Napoli P, Rappa F, et al. Lymph node metastasis in lower lip squamous cell carcinoma in relation to tumour size, histologic variables and p27Kip1 protein expression. Oral Oncol. 2004;40:92–8. https://doi.org/10.1016/S1368-8375(03)00141-6.
Umeda M, Yokoo S, Take Y, Omori A, Nakanishi K, Shimada K. Lymph node metastasis in squamous cell carcinoma of the oral cavity: correlation between histologic features and the prevalence of metastasis. Head Neck. 1992;14:263–72. https://doi.org/10.1002/hed.2880140402.
Franceschi D, Gupta R, Spiro RH, Shah JP. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg. 1993;166:360–5. https://doi.org/10.1016/S0002-9610(05)80333-2.
Shin JH, Yoon HJ, Kim SM, Lee JH, Myoung H. Analyzing the factors that influence occult metastasis in oral tongue cancer. J Korean Assoc Oral Maxillofac Surg. 2020;46:99–107. https://doi.org/10.5125/jkaoms.2020.46.2.99.
Frierson HF Jr, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol. 1986;17:346–54. https://doi.org/10.1016/S0046-8177(86)80457-9.
Sparano A, Weinstein G, Chalian A, Yodul M, Weber R. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004;131:472–6. https://doi.org/10.1016/j.otohns.2004.04.008.
Kurokawa H, Yamashita Y, Takeda S, Zhang M, Fukuyama H, Takahashi T. Risk factors for late cervical lymph node metastases in patients with stage I or II carcinoma of the tongue. Head Neck. 2002;24:731–6. https://doi.org/10.1002/hed.10130.
Jakobsson PA, Eneroth CM, Killander D, Moberger G, Mårtensson B. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys Biol. 1973;12:1–8. https://doi.org/10.3109/02841867309131085.
Willén R, Nathanson A. Squamous cell carcinoma of the gingiva Histological classification and grading of malignancy. Acta Oto-laryngol. 1973;75:299–300. https://doi.org/10.3109/00016487309139722.
Yamane M, Ishii J, Izumo T, Nagasawa T, Amagasa T. Noninvasive quantitative assessment of oral tongue cancer by intraoral ultrasonography. Head Neck. 2007;29:307–14. https://doi.org/10.1002/hed.20523.
Kaneoya A, Hasegawa S, Tanaka Y, Omura K. Quantitative analysis of invasive front in tongue cancer using ultrasonography. J Oral Maxillofac Surg. 2009;67:40–6. https://doi.org/10.1016/j.joms.2007.08.006.
Shinozaki Y, Jinbu Y, Ito H, Noguchi T, Kusama M, Matsumoto N, et al. Relationship between appearance of tongue carcinoma on intraoral ultrasonography and histopathologic findings. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:634–9. https://doi.org/10.1016/j.oooo.2014.02.001.
Chien CY, Su CY, Hwang CF, Chuang HC, Chen CM, Huang CC. High expressions of CD105 and VEGF in early oral cancer predict potential cervical metastasis. J Surg Oncol. 2006;94:413–7. https://doi.org/10.1002/jso.20546.
Lim SC, Zhang S, Ishii G, Endoh Y, Kodama K, Miyamoto S, et al. Predictive markers for late cervical metastasis in stage I and II invasive squamous cell carcinoma of the oral tongue. Clin Cancer Res. 2004;10:166–72. https://doi.org/10.1158/1078-0432.CCR-0533-3.
Gontarz M, Wyszyńska-Pawelec G, Zapała J, Czopek J, Lazar A, Tomaszewska R. Immunohistochemical predictors in squamous cell carcinoma of the tongue and floor of the mouth. Head Neck. 2016;38(Suppl 1):E747–53. https://doi.org/10.1002/hed.24087.
Mermod M, Jourdan EF, Gupta R, Bongiovanni M, Tolstonog G, Simon C, et al. Development and validation of a multivariable prediction model for the identification of occult lymph node metastasis in oral squamous cell carcinoma. Head Neck. 2020;42:1811–20. https://doi.org/10.1002/hed.26105.
Shan J, Jiang R, Chen X, Zhong Y, Zhang W, Xie L, et al. Machine learning predicts lymph node metastasis in early-stage oral tongue squamous cell carcinoma. J Oral Maxillofac Surg. 2020;78:2208–18. https://doi.org/10.1016/j.joms.2020.06.015.
Yip SS, Aerts HJ. Applications and limitations of radiomics. Phys Med Biol. 2016;61:R150–66. https://doi.org/10.1088/0031-9155/61/13/R150.
Acknowledgements
We thank Dr. Noriaki Takeda and Dr. Makoto Fukui for their scientific advice.
Funding
This work was supported by Grants-in-Aid for Scientific-Research (Grant number 19K10268).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by TK, AH, KK, AT, MS, YK, HI, and YM. The first draft of the manuscript was written by TK, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study was approved by the ethics committee of the Tokushima University (approval number 3212, date of approval July 23, 2018), and the study protocol was performed in accordance to the Declaration of Helsinki.
Informed consent
The requirement for informed consent was waived by the institutional review board owing to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kudoh, T., Haga, A., Kudoh, K. et al. Radiomics analysis of [18F]-fluoro-2-deoxyglucose positron emission tomography for the prediction of cervical lymph node metastasis in tongue squamous cell carcinoma. Oral Radiol 39, 41–50 (2023). https://doi.org/10.1007/s11282-022-00600-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11282-022-00600-7