Abstract
Two strains of Yarrowia lipolytica (CBS 2075 and DSM 8218) were first studied in bioreactor batch cultures, under different controlled dissolved oxygen concentrations (DOC), to assess their ability to assimilate aliphatic hydrocarbons (HC) as a carbon source in a mixture containing 2 g·L−1 of each alkane (dodecane and hexadecane), and 2 g·L−1 hexadecene. Both strains grew in the HC mixture without a lag phase, and for both strains, 30 % DOC was sufficient to reach the maximum values of biomass and lipids. To enhance lipid-rich biomass and enzyme production, a pulse fed-batch strategy was tested, for the first time, with the addition of one or three pulses of concentrated HC medium. The addition of three pulses of the HC mixture (total of 24 g·L−1 HC) did not hinder cell proliferation, and high protease (> 3000 U·L−1) and lipids concentrations of 3.4 g·L−1 and 4.3 g·L−1 were achieved in Y. lipolytica CBS 2075 and DSM 8218 cultures, respectively. Lipids from the CBS 2075 strain are rich in C16:0 and C18:1, resembling the composition of palm oil, considered suitable for the biodiesel industry. Lipids from the DSM 8218 strain were predominantly composed of C16:0 and C16:1, the latter being a valuable monounsaturated fatty acid used in the pharmaceutical industry. Y. lipolytica cells exhibited high intrinsic surface hydrophobicity (> 69 %), which increased in the presence of HC. A reduction in surface tension was observed in both Y. lipolytica cultures, suggesting the production of extracellular biosurfactants, even at low amounts. This study marks a significant advancement in the valorization of HC for producing high-value products by exploring the hydrophobic compounds metabolism of Y. lipolytica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aliphatic hydrocarbons (HC) (e.g., alkanes and alkenes) have diverse applications in industry and are used as raw material for the synthesis of various chemicals, liquid transportation fuels (e.g., gasoline, diesel, and jet fuel), lubricants, and plastics (Kuppusamy et al. 2020). As one of the higher n-alkanes, n-dodecane is often employed as the surrogate or a surrogate component for kerosene, jet fuel, and diesel (Mao et al. 2020). Hexadecane is one of the major components of diesel fuel, ranging from a few percent to over 10 %, depending on factors such as the refinery process used and additives included in the fuel formulation (Krummenacher et al. 2003). This aliphatic hydrocarbon is also one of the predominant alkanes present in hydrotreated vegetable oil, which is used as a biofuel (Tang et al. 2018). Furthermore, hexadecane is used as a base material in the production of most commercial organic lubricants (Cafolla and Voïtchovsky 2020). Hexadecene, a linear alpha-olefin, is widely used as a raw material for chemical products such as synthetic plasticizers, synthetic detergents, and lubricants (Yang et al. 2024).
Due to the broad use of the above-mentioned HC in high volumes in many products and formulations is inevitable that they are transferred to wastewater treatment plants or soil. Considering the important role of aliphatic hydrocarbons in chemical synthesis and industrial production and the environmental impact owing to their prevalence in terrestrial and aquatic ecosystems, its research has garnered increasing attention over the last decades. To minimize or eradicate environmental pollution by HC contaminants, several physical, chemical, and biological approaches have been studied. Among those strategies, biological treatments are considered one of the most promising eco-friendly options for highly HC-polluted effluents. Microorganisms use these compounds as carbon sources and, in some cases, produce added-value metabolites while reducing organic load and environmental impact (Thorat and Sonwani 2022). However, most relevant studies are focused on the bacteria species (e.g., Acinetobacter sp., Bacillus sp., Pseudomonas sp., Sphingomonas sp., Rhodococcus sp., and Alcaligenes sp.) owing to their ability to utilize such compounds as energy sources and their potential to bioremediate HC-polluted streams (Imron et al. 2020; Ławniczak et al. 2020; Kebede et al. 2021).
Yarrowia lipolytica is commonly found in oil-contaminated environments and demonstrates a notable affinity for hydrophobic substrates, including aliphatic HC (Lopes et al. 2022; Miranda et al. 2024) and aromatic compounds (Ferreira et al. 2023). Y. lipolytica developed strategies to increase the bioavailability of HC, including the production of extracellular biosurfactants (Ferreira et al. 2023). Additionally, yeast cells can directly adhere to HC droplets due to their hydrophobic cell surfaces (Amaral et al. 2006). Upon entering the cells, alkanes are metabolized via the β-oxidation pathway into acetyl-CoA, which can be incorporated in the glyoxylate, Krebs, or methyl-citrate cycles (Fukuda 2023).
Yarrowia lipolytica has garnered considerable scientific interest as a noteworthy cell factory for the secretion of valuable compounds, namely enzymes (Janek et al. 2020), organic acids (Tomaszewska-Hetman et al. 2021), and polyols (Tomaszewska et al. 2014; Rywińska et al. 2024). Additionally, it has demonstrated excellence as a producer of lipids (Papanikolaou and Aggelis 2010; Lazar et al. 2014; Rakicka et al. 2015; Dobrowolski et al. 2019), and recent work (Miranda et al. 2024) proved the ability of the CBS 2075 strain to produce lipid-rich biomass and protease from hexadecane. Y. lipolytica DSM 8218, formerly known as C. lipolytica, was isolated from fuel storage tanks and possesses a specific ability to metabolize aromatic hydrocarbons such as naphthalene, biphenyl, and benzo(a)pyrene (Cerniglia and Crow 1981). This study intended to test both Y. lipolytica strains in a mixture of higher alkanes (dodecane and hexadecane) and alkenes (hexadecene) since no papers have been published so far concerning their application to produce lipids and enzymes from such HC mixture. Firstly, batch cultures were carried out in a lab-scale stirred-tank bioreactor under different controlled dissolved oxygen concentrations (DOC) (30 – 90%) to select the best oxygenation conditions for HC assimilation and biomass, lipids, and enzymes (protease and lipase) production. As a prospect to improve lipid-rich biomass and extracellular enzyme production by both Y. lipolytica strains, a pulse fed-batch approach was attempted at 30 % DOC. In this strategy, intermittent feeding of concentrated HC medium (by one or three pulses) after HC depletion was applied as a strategy to supply more substrate to the yeast cells preventing the potential inhibitory effect of high initial HC concentration.
Materials and methods
Yeast strains preservation and inoculum preparation
Yarrowia lipolytica CBS 2075 and Y. lipolytica DSM 8218 grew overnight in YPD medium (20 g·L−1 glucose, 20 g·L−1 peptone, and 10 g·L−1 yeast extract) to prepare cryo-stocks in sterile microtubes (800 μL of yeast culture and 200 μL of pure glycerol), which were preserved at − 80 °C. Pre-inoculum cultures (150 mL of YPD medium) were inoculated with a thawed culture of one microtube and placed in an orbital incubator at 27 °C and 200 rpm. After 16 h of growth, yeast cells were harvested by centrifugation and resuspended in the culture medium at an initial dry cell concentration of 0.5 g·L−1.
Batch cultures
Batch cultures were carried out in 2-L stirred tank bioreactors (DASGIP Parallel Bioreactor System, Eppendorf, Germany) with 1.2-L of medium composed of 6 g·L−1 HC mixture (2 g·L−1 dodecane, 2 g·L−1 hexadecane, and 2 g·L−1 hexadecene), 0.5 g·L−1 ammonium sulfate, and 3.4 g·L−1 corn steep liquor (CSL, C4648-500G, Sigma-Aldrich®) — initial C/N ratio of 48. The experiments were conducted at 27 °C and pH of 5.5 ± 0.5 automatically controlled by the addition of NaOH 2 M or HCl 2 M. To select the best controlled DOC conditions for biomass, lipids and enzyme production by Y. lipolytica strains, four conditions of controlled DOC (30 %, 50 %, 70 %, and 90 % of saturation) were studied through a cascade control mode, in which the stirring rate automatically varied between 200 rpm and 800 rpm. The specific airflow rate was set at 3 vvm (90 % DOC), 2 vvm (70 % DOC), and 1 vvm (50 % and 30 % DOC). DOC was monitored with a polarographic oxygen probe (Inpro6820/12/320-type, Mettler Toledo).
Pulse fed-batch cultures
Yarrowia lipolytica strains grew for 48 h in a medium comprising 6 g·L−1 of an HC mixture (2 g·L−1 dodecane, 2 g·L−1 hexadecane and 2 g·L−1 hexadecene), 0.5 g·L−1 ammonium sulfate, and 3.4 g·L−1 CSL (batch stage) — initial C/N ratio of 48, followed by the addition of a single pulse of concentrated medium to attain the initial concentration of each compound. Additional experiments were performed in which batch phase (24 h) was followed by adding three pulses of concentrated medium at 24 h, 48 h, and 72 h. All experiments were carried out at a specific airflow rate of 1 vvm and controlled DOC of 30 % by automatic variation of stirring rate between 200 rpm and 500 rpm.
Analytical methods
Samples were collected at specific intervals to quantify biomass, HC concentration, extracellular enzyme activity, cell hydrophobicity, and surface tension of the medium. Yeast cells were harvested by centrifugation and stored at − 20 °C for further quantification of intracellular lipids and long-chain fatty acids composition.
Biomass concentration was quantified by the optical density of cultures at 600 nm and converted to cell dry weight (g·L−1) using a calibration curve for each yeast strain. Extracellular lipase activity was quantified in samples supernatant according to Miranda et al. (2024), using p-nitrophenyl butyrate 1 mM as substrate dissolved in 250 μL of a mixture with phosphate buffer 50 mM (pH 7.3), acetone 4 % (v/v), and Triton-X 4 % (v/v), and following the enzymatic reaction for 10 min at 37 °C. Lipase activity was calculated by linear regression of absorbance versus time, using the molar extinction coefficient of p-nitrophenol (2.3 mM−1). One unit of activity was expressed as the amount of lipase that produces 1 μmol of p-nitrophenol per minute under the conditions used. Extracellular protease activity was measured in the culture supernatant using 0.5 % (w/v) azocasein dissolved in sodium acetate buffer 50 mM (pH 5) as substrate at 37 °C for 40 min. One unit of enzyme activity was defined as the amount of enzyme that causes an increase of 0.01 absorbance comparatively to the blank (supernatant was replaced by sodium acetate buffer 50 mM) per minute under assay conditions (Miranda et al. 2024).
The hydrophobicity of the cells’ surface was evaluated by the microbial adhesion to hydrocarbons (MATH) test (Rosenberg et al. 1980). Yeast cells were harvested, centrifuged (8000 rpm, 10 min), washed with phosphate buffer 50 mM (pH 7), centrifuged, and resuspended in the same buffer to an optical density of approximately 0.7 (A0). In a glass tube, 1.5 mL of cellular suspension was mixed with 1.3 mL of hexadecane, vortex-mixed for 1 min, and left to rest for 9 min to obtain a separation of the two phases. The aqueous phase containing cells was transferred to a tube and the absorbance at 600 nm (A) was measured. The results are given as the percentage of adhesion: % adhesion = 1 − (A/A0).
The surface tension (mN·m−1) was quantified using cell-free supernatant with a Tensiometer K20 (KRÜSS GmbH, Hamburg, Germany) at room temperature, according to Du Noüy ring method (Gudiña et al. 2012). Before quantification, the equipment was calibrated with ultra-pure water, and all measurements were performed in triplicate.
Microbial lipids were quantified in lyophilized cells by the phospho-vanillin colorimetric method as described by Lopes et al. (2019). Results were expressed as microbial lipids content (mass of lipids per gram of cell dry weight, %) and microbial lipids concentration (by multiplying the lipids content by biomass concentration in the medium). The analysis of fatty acids composition was performed by gas chromatography after extraction of fatty acids from lyophilized cells with chloroform and further methylation using a mixture of methanol acidified with sulfuric acid (85:15, v/v), and pentadecanoic acid was used as the internal standard (Pereira et al. 2021). The relative amount of each fatty acid (%, w/w) was determined as the ratio between its concentration (g·L−1) and the sum of the concentrations of all fatty acids identified in the sample.
HC were extracted by liquid–liquid extraction using hexane as solvent (1:6, v/v) and undecane (C11) as the internal standard and quantified by gas chromatography using helium as the carrier gas at 1 mL·min−1. The temperature of the injector and FID detector were, respectively, 285 °C and 300 °C. The initial temperature of the oven was maintained at 60 °C for 1 min, followed by a gradual increase at a rate of 8 °C per minute until reaching 300 °C. The system was maintained at 300 °C for 5 min and then gradually decreased to 60 °C at a rate of 40 °C per minute (Miranda et al. 2024).
Statistical analysis
All data represent the mean of two independent replicates. Statistical analysis was performed with Statgraphics Centurion XVI Version 16.2.04 (StatPoint Technologies Inc., USA), using one-way analysis of variance (ANOVA) and Tukey’s multi-range test to identify statistically significant differences in mean values (95 % level of confidence).
Results
Batch cultures
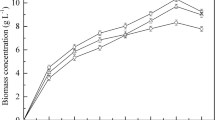
Yarrowia lipolytica CBS 2075 and Y. lipolytica DSM 8218 grew in a 6 g·L−1 HC mixture (2 g·L−1 hexadecane, 2 g·L−1 hexadecene, and 2 g·L−1 dodecane) without inhibition and no lag phase. Regardless of the yeast strain, DOC in the 30 % to 90% saturation had no significant effect on cell growth (Fig. 1) and biomass yield (Table 1). In all DOC conditions, a specific growth rate of approximately 0.16 h−1 and 0.14 h−1 was obtained in the exponential phase (first 8 h of growth) of CBS 2075 and DSM 8218. The final biomass concentration and biomass yield achieved in Y. lipolytica CBS 2075 cultures were, approximately, twofold higher than those obtained with the DSM 8218 strain.
Biomass concentration (closed symbols) and total hydrocarbon consumption (open symbols) obtained in batch cultures of (a) Y. lipolytica CBS 2075 and (b) Y. lipolytica DSM 8218 with different controlled dissolved oxygen concentrations (% of saturation): 90 (▼,▽), 70 (●, ○), 50 (▲, ∆) and 30 (■, □). The error bars represent the standard deviation of two independent replicates
In this work, the global uptake rate of HC (RHC) by Y. lipolytica CBS 2075 was similar in the range of DOC tested (Table 1), and all HC were assimilated before the end of cultivation time (Fig. 1a). Likewise, Y. lipolytica DMS 8218 also assimilated all HC in the first 32 h (Fig. 1b). Dodecane, hexadecane, and hexadecene were assimilated at similar uptake rates in all DOC conditions in Y. lipolytica CBS 2075 cultures. However, dodecane assimilation by Y. lipolytica DSM 8218 in 30 % DOC cultures was, approximately, fivefold lower than the uptake rate of hexadecane and hexadecane.
Generally, in both Y. lipolytica cultures, the lipids content decreased from 24 h to 72 h. However, this decrease was only statistically significant at 90 % of DOC (Fig. 2). Due to the high biomass production in cultures of Y. lipolytica CBS 2075, the maximum lipids concentration (g·L−1) achieved with this strain was higher, regardless of the DOC studied. Specifically, at 30 % DOC, the concentration of the lipids was 44 % higher than that obtained in cultures of Y. lipolytica DSM 8218. Regardless of the yeast strain, DOC did not affect lipid yield (YL/S) but higher YL/S were reached with the CBS 2075 strain. Particularly at 30 % DOC, the lipid yield in the CBS 2075 culture was 34 % higher than that obtained in the Y. lipolytica DSM 8218 culture. In general, the volumetric lipid productivity (QL) was higher in CBS 2075 cultures and DOC had a distinct effect depending on yeast strains. The lowest value of QL was obtained at 30 % DOC in CBS 2075 cultures, whereas for DSM 8218 the minimum was attained at 70 % DOC. A 2.4-fold decrease in the specific rate of lipids synthesis (qL) was observed in CBS 2075 culture by decreasing DOC from 90 % to 30 %. In DSM 8218 cultures, the highest qL was reached at 90 % DOC (Table 1).
Lipids content (%) of (a) Y. lipolytica CBS 2075 and (b) Y. lipolytica DSM 8218 cells at 24 h (white bars), 48 h (black bars), and 72 h (grey bars) obtained in different conditions of controlled dissolved oxygen concentration (DOC, % saturation). The error bars represent the standard deviation of two independent replicates. Bars with the same letter do not present statistically significant differences (p ≥ 0.05)
Regardless of the DOC, higher proteolytic activities were obtained in Y. lipolytica CBS 2075 cultures compared to the DSM 8218 strain (Table 2). In CBS 2075 cultures, DOC had no significant effect on protease activity, reaching the maximum value at 28 h, which coincided with the stationary growth phase, remaining constant until the end. By contrast, an enhancement in protease was observed by increasing DOC from 30 % to 90 % in Y. lipolytica DSM 8218 cultures. However, the production profile was similar, and the maximum activity obtained at 24 h lasted constant until the end of cultivation.
In Y. lipolytica CBS 2075 cultures, an eightfold improvement in lipolytic activity was observed by increasing the DOC from 30 % to 90 %. In Y. lipolytica DSM 8218 cultures, lipase secretion was observed only at 90 % DOC (Table 2). Regardless of yeast strain and DOC conditions, the maximum activity was obtained at 8 h of cultivation, after which a decay in activity was observed.
Pulse fed-batch cultures
HC consumption and production of lipids and protease
The results obtained in batch cultures demonstrated that both Y. lipolytica strains can assimilate 6 g·L−1 of HC mixture, directing them towards lipid-rich biomass and extracellular protease production. Moreover, the cell growth reached the stationary phase after 24 h (DSM 8218 strain) and 32 h (CBS 2075 strain), coinciding with the total assimilation of HC. To achieve high biomass concentration while avoiding potential inhibitory effects by increasing the initial HC concentration, several experiments were carried out with the addition of an HC mixture with one pulse at 48 h or with three pulses at 24 h, 48 h, and 72 h.
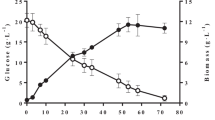
The maximum biomass concentration attained in CBS 2075 and DSM 8218 cultures after one pulse addition (Fig. 3a, c) was 57 % and 59 % higher, respectively than that obtained in batch cultures (Fig. 1). While a significant improvement (p < 0.05) in global biomass yield was observed in CBS 2075 cultures with the addition of one pulse, no significant effect was noted for the DSM 8218 strain when compared to batch cultures. Following the pulse addition, both yeast strains entered a second exponential growth phase only 4 h after the pulse. The addition of an HC pulse enhanced biomass production rate in CBS 2075 cultures, and an increase from (0.22 ± 0.01) g·L−1·h−1 (batch stage of 48 h) to (0.29 ± 0.01) g·L−1·h−1 was achieved after the pulse addition (Fig. 3a). By contrast, in DSM 8281 cultures, no significant differences were found in the biomass production rate during 1st batch stage (0.16 ± 0.03) g·L−1·h−1 and after the pulse addition (0.17 ± 0.02) g·L−1·h−1 (Fig. 3c).
Biomass concentration (closed symbols) and total hydrocarbons consumption (open symbols) obtained in pulse fed-batch cultures of Y. lipolytica CBS 2075 (a, b) and Y. lipolytica DSM 8218 (c, d) with one pulse (●, ○) and three pulses (■, □). The error bars represent the standard deviation of two independent replicates. The arrows indicate the moment at which a pulse of concentrated HC-mixture was added to the culture
After the pulse addition, all HC were completely assimilated by both Y. lipolytica strains in 32 h, coinciding with the beginning of a second stationary growth phase (Fig. 3a, c). Due to the high biomass concentration at the time of the pulse addition (48 h), faster assimilation of HC was observed compared to the 1st batch phase. For Y. lipolytica CBS 2075, the global uptake rate increased from (0.20 ± 0.02) g·L−1·h−1 to (0.224 ± 0.002) g·L−1·h−1 with the pulse addition (Fig. 3a). With DSM 8218, an increase from (0.19 ± 0.02) g·L−1·h−1 to (0.23 ± 0.02) g·L−1·h−1 was attained (Fig. 3c).
An improvement in maximum biomass concentration of 40 % and 52 % was obtained in experiments with three pulses with CBS 2075 and DSM 8218 strains, respectively, compared to experiments with only one pulse. Both yeast strains assimilated a total amount of 24 g·L−1 HC, in total of the 1st batch phase and three pulses. Moreover, the maximum biomass concentration of CBS 2075 and DSM 8218 cultures with three pulses (Fig. 3b, d) was, respectively, 74 % and 80 % higher than that attained in batch cultures (Fig. 1). It is noteworthy that pulse fed-batch cultures enable the maintenance of a high number of viable cells since cells of Y. lipolytica promptly entered a new exponential phase after each pulse (Fig. 3b, d), resulting in an increased biomass production rate. In CBS 2075 cultures, a significant increase in biomass production rate was achieved with the addition of pulses compared to the 1st batch phase (24 h). A biomass production rate of (0.23 ± 0.01) g·L−1·h−1 was attained in the first 24 h of cultivation, and after the 1st and 2nd pulses this value increased to (0.30 ± 0.01) g·L−1·h−1 and (0.33 ± 0.01) g·L−1·h−1, respectively. After the 3rd pulse, a remarkable biomass production rate of (0.55 ± 0.02) g·L−1·h−1 was attained since 4.7 g·L−1 of biomass was produced in only 8 h (from 72 h to 80 h of cultivation) (Fig. 3b). Regarding DSM 8218 cultures, a biomass production rate of (0.16 ± 0.01) g·L−1·h−1 was attained within 24 h (1st batch), and no significant differences were found in the biomass production rate (0.19 ± 0.02) g·L−1·h−1) reached after 1st pulse. However, biomass production rate increased after the 2nd (0.38 ± 0.03) g·L−1·h−1) and 3rd (0.50 ± 0.04) g·L−1·h−1) pulses, being 2.4-fold and 3.1-fold higher than that obtained in the 1st batch (Fig. 3d). The global biomass yield was similar in Y. lipolytica DSM 8218 cultures, regardless of the number of pulses, but a slight decrease in biomass yield was observed for CBS 275 strain by adding three pulses of HC (Table 3). As was observed in batch cultures, higher biomass concentration was reached in Y. lipolytica CBS 2075 cultures than in Y. lipolytica DSM 8218 cultures (Fig. 3).
In experiments with a single pulse, adding an HC-mixture did not enhance the lipids content (%, w/w), which remained similar throughout the cultivation period in both CBS 2075 and DSM 8218 cultures (Fig. 4a, b). In contrast, in experiments with three pulses, the lipids content attained at 72 h (after two pulses addition) in CBS 2075 cultures was 1.4-fold higher than that obtained at 24 h (batch phase) (Fig. 4a). In the DSM 8218 culture, there was a 1.7-fold increase in lipids content at 96 h (after three pulses addition), compared to that achieved at 24 h (1st batch cycle) (Fig. 4b).
Lipids content (%) of (a) Y. lipolytica CBS 2075 and (b) Y. lipolytica DSM 8218 cells at 24 h, 48 h, 72 h, and 96 h obtained in pulse fed-batch cultures. The error bars represent the standard deviation of two independent replicates. Bars with the same letter for each yeast strain do not present statistically significant differences (p ≥ 0.05)
In pulse fed-batch experiments, a significant improvement in maximum lipids concentration was achieved (Table 3) compared to those obtained in batch cultures (Table 1), owing to the high biomass concentration reached. By contrast, YL/S, QL, and qL were overall similar to the values obtained in batch cultures, regardless of the Y. lipolytica strain.
In experiments with a single pulse, a three and twofold enhancement in maximum lipids concentration was obtained in cultures of CBS 2075 and DSM 8218, respectively, compared to those reached in batch cultures. Furthermore, three pulses led to a five and sevenfold improvement in maximum lipids concentration for CBS 2075 and DSM 8218 cultures, respectively, compared to cultures without pulses. The increase in the number of pulses from one to three led to a clear enhancement of the maximum lipid production by both strains (Table 3). The maximum lipids concentration attained in CBS 2075 and DSM 8218 cultures was, respectively, 42 % and 71 % higher than that reached with one pulse. The amount of lipids produced by Y. lipolytica DSM 8218 in the experiments with one pulse and three pulses were, respectively, 60 % and 21 % lower than those reached in cultures with CBS 2075 strain (Table 3). In CBS 2075 cultures, the lipid yield was statistically equal for both pulse strategies, whereas a twofold increase was reached in DSM 8218 cultures by increasing the number of pulses (Table 3). In addition, YL/S values reached with the CBS 2075 strain were higher than those obtained in cultures of Y. lipolytica DSM 8218. Particularly in experiments with one pulse, the lipid yield was 66 % higher than that obtained in Y. lipolytica DSM 8218 cultures. A two and fivefold increase in the volumetric lipid productivity was obtained in CBS 2075 and DSM 8218 cultures, respectively, by increasing the number of pulses. Whereas no significant differences were found in the specific rate of lipids synthesis for CBS 2075 cultures, a 2.2-fold increase was obtained in DSM 8218 cultures with three pulses (Table 3).
Lipids accumulated by Y. lipolytica CBS 2075 in both pulses’ strategies were predominantly composed of palmitic (C16:0) and oleic (C18:1) acids, followed by linoleic (C18:2) and palmitoleic (C16:1) acids, with low amounts of stearic (C18:0) and cis-10-heptadecenoic (C17:1) acids (around 5 % for both cases) (Table 4). In experiments with one pulse, strain DSM accumulated lipids rich in palmitic (C16:0), palmitoleic (C16:1), and linoleic (C18:2) acids, but lipids produced after the addition of three pulses were predominantly composed of palmitic (C16:0) and palmitoleic (C16:1) acids. Moreover, in cultures with three pulses, the content of C16:0 was 1.5-fold higher, while the content of C18:2 was 1.7-fold lower than those attained with one pulse (Table 4). Regardless of the pulse strategy, Y. lipolytica DSM 8218 accumulated around three times lower C18:1 than CBS 2075 strain. The unsaturated/saturated fractions of lipids produced by CBS 2075 and DSM 8218 strains were similar for the same pulse strategy studied, but lower unsaturated fractions were attained by increasing the number of pulses, regardless of the Y. lipolytica strain (Table 4).
The protease production significantly increased by changing the cultivation mode from batch to pulse fed-batch, particularly for the DSM 8218 strain (Table 2). Protease activities reached in cultures performed with one pulse and three pulses were, respectively, 3- and 6-fold (strain CBS 2075), and 10- and 23-fold (strain DMS 8218) higher than those attained in batch cultures. Moreover, a twofold improvement in maximum protease activity was attained for both Y. lipolytica strains by increasing the number of pulses from one to three (Table 2). It is worth noticing that in pulse fed-batch, protease production by the DSM 8218 strain was similar to that obtained for the CBS 2075 strain, contrary to what was observed in batch cultures (Table 2). Regardless of the yeast strain and pulse fed-batch strategy, protease production followed a similar pattern to batch cultures, being the maximum value obtained at the beginning of the stationary growth phase (growth-associated production). Furthermore, after each HC addition, protease production accompanied the new exponential growth phase and increased until it reached the stationary phase.
Cells hydrophobicity and surface tension of the medium
Table 4 shows the surface tension of cells-free supernatant and hydrophobicity of Y. lipolytica CBS 2075 and DSM 8218 cells at 0 h and 96 h. Regardless of pulse fed-batch strategy, the surface tension of the medium significantly decreased in CBS 2075 and DSM 8218 supernatants.
In the presence of HC, the hydrophobicity of CBS 2075 cells increased by approximately 12 % and 10 % in experiments with one and three pulses, respectively, after 96 h of cultivation. For strain DSM 8218, an increase of approximately 10 % was observed with one pulse and three pulses, compared to the hydrophobicity of yeast cells grown in glucose (Table 5).
Discussion
In this work, DOC had no significant effect on cell growth (Fig. 1) and biomass yield (Table 1) in cultures of Y. lipolytica CBS 2075 and Y. lipolytica DSM 8218. However, according to Fukuda (2023), the metabolic pathway of n-alkanes in Y. lipolytica involves a final step where fatty acids are activated to acyl-CoAs. Subsequently, these molecules undergo metabolism into acetyl-CoA through β-oxidation, a metabolic pathway that is highly reliant on a sufficient oxygen supply. Earlier studies showed that Y. lipolytica CBS 2075 assimilated 5 g·L−1 of hexadecane within 24 h at 600 rpm, enabling the maintenance of DOC nearly to 90 % of saturation (Miranda et al. 2024). It is noteworthy that the global uptake rate of HC obtained in this study surpasses those reported in the literature for bacterial species utilizing hexadecane at flask-scale (Tzintzun-Camacho et al. 2012; Zhong et al. 2014; Castro et al. 2017; Noveiri et al. 2023), which are the microbial species extensively studied for the biodegradation of HC.
In literature, the effect of DOC on lipids production by Y. lipolytica is far from consensual. Additionally, there are no studies in the literature reporting the impact of DOC on lipids production by Y. lipolytica strains from an HC mixture. Some authors recognize the significance of low oxygen availability for lipids synthesis by Y. lipolytica from both hydrophobic and hydrophilic substrates (Papanikolaou et al. 2002, 2007; Lopes et al. 2018, 2019; Pereira et al. 2021; Fabiszewska et al. 2022), while other researchers reported that a high lipid content is achieved in highly aerated cultures (Bellou et al. 2014; Fabiszewska et al. 2021). Under DOC of 90 %, the reduction in lipids content likely occurred due to intracellular lipids mobilization (lipids turnover) (Fig. 2), coinciding with the total depletion of the HC mixture observed at 24 h (Fig. 1). According to some authors, the mobilization of the intracellular lipids in Y. lipolytica strains appears to be associated with the complete depletion of the carbon sources from the medium (Dias et al. 2023). Furthermore, highly aerated cultures seem to enhance lipid mobilization inside the yeast cells (Magdouli et al. 2018).
Overall, the values of YL/S, QL, and qL were similar in batch and pulse fed-batch cultures, regardless of the Y. lipolytica strain (Tables 1, 3). The lipid yields obtained in this study are comparable to those found in the literature for Y. lipolytica KKP 379 and engineered Y. lipolytica AJD pAD-DGA1 growing in olive oil, glucose, and crude glycerol (Dobrowolski et al. 2019; Fabiszewska et al. 2021). Moreover, they are higher than those observed for Y. lipolytica cultures in glucose (Carsanba et al. 2020) and volatile fatty acids (Llamas et al. 2020). The QL values obtained herein were similar to those achieved by Cryptococcus psychrotolerans IITRFD growing in aromatic hydrocarbons (naphthalene, anthracene, and pyrene) (Deeba et al. 2018). Although in this study DOC had no significant effect on lipid yield, in fed-batch cultures of lipid-engineered Y. lipolytica JMY4086 with crude glycerol as substrate, higher values of YL/S and QL were reached under unregulated oxygen conditions, compared to experiments carried out at 50 % controlled DOC and highly aerated cultures (Rakicka et al. 2015). By contrast, in cultures supplemented with waste fish oil, a significant increase in the values of YL/S and QL was reached with controlled DOC (maintained above 20 % of saturation by varying agitation rate) since higher quantities of lipids were produced by Y. lipolytica KKP 379 (Fabiszewska et al. 2021). Regardless of the yeast strain, the specific rates of lipids synthesis (qL) obtained herein were similar or even higher compared with that reported by other Y. lipolytica strains growing in glucose/stearin mixture (Papanikolaou et al. 2006) and olive oil (Vasiliadou et al. 2018).
Lipids accumulation is intrinsically correlated with citric acid production in Y. lipolytica cultures since nitrogen-limited conditions (higher C/N ratio) are simultaneously required for lipids and citric acid synthesis. Given that, the synthesis of lipids can redirect metabolism away from citric acid production, and vice versa (Papanikolaou et al. 2020). By contrast, other authors found citric acid synthesis by Y. lipolytica independent of the C/N ratio (Ferreira et al. 2016; Cavallo et al. 2020). Although works were reporting the production of citric acid by Y. lipolytica from n-paraffin (Crolla and Kennedy 2004) and hexadecane (Finogenova et al. 2005), citric acid was not secreted in this study, suggesting that microbial metabolism was toward the accumulation of lipids alternatively to the synthesis of citric acid.
In CBS 2075 cultures, DOC had no significant effect on protease activity as already observed for this yeast strain when cultured in hexadecane as the sole carbon source (Miranda et al. 2024). In this context, it is noteworthy that low DOC values are suitable for achieving high proteolytic activities, which is significant for potentially lowering operational costs during the scale-up of the bioprocess, as it implies lower power input requirements. As observed in this study for Y. lipolytica DSM 8218, an increase in protease activity under high DOC was also attained in cultures of Y. lipolytica W29 growing in glucose (Lopes et al. 2009) and waste cooking oils (Lopes et al. 2019).
High oxygenation conditions have been shown to enhance lipase synthesis by Y. lipolytica growing in waste oils (Alonso et al. 2005; Snopek et al. 2021) and olive oil (Lopes et al. 2008). It seems that the activity of the LIP2 promoter, responsible for the secretion of the main extracellular lipase in Y. lipolytica, decreases under low DOC (Kar et al. 2010).
To enhance lipid-rich biomass and extracellular enzyme production in both Y. lipolytica strains, a pulse fed-batch strategy was studied at 30 % DOC, in which one or three pulses of concentrated HC medium were applied. It should be highlighted that both yeast strains consumed a total of 12 g·L−1 of HC in 80 h (Fig. 3), as a result of a single pulse addition at 48 h, which is a remarkable and uncommon feature in other microbial species (Zhong et al. 2014; Castro et al. 2017; SadrAzodi et al. 2019).
The results presented in this work suggest that three pulses of concentrated HC medium were an effective strategy to enhance the amount of lipids accumulated by Y. lipolytica strains. The maximum lipids concentration obtained herein for both Y. lipolytica strains, ranging from 1 g·L−1 to 4.3 g·L−1, are comparable or even higher than those found in the literature for Y. lipolytica growing in hexadecane (Miranda et al. 2024), pentadecane and heptadecane (Matatkova et al. 2017), volatile fatty acids (Pereira et al. 2021, 2022; Morales-Palomo et al. 2022), vegetable oils refinery wastewaters (Louhasakul and Cheirsilp 2013; Darvishi et al. 2019; Sarris et al. 2019), or synthetic medium mimicking lignocellulosic biomass hydrolysate (Dias et al. 2023).
In this study, the fatty acids composition of lipids was highly dependent on Y. lipolytica strain (Table 4). Lipids produced by Y. lipolytica CBS 2075 from hexadecane (Miranda et al. 2024) were richer in C16:0 and C16:1, but considerably lower amounts of C18:1 (17 %) and C18:2 (8 %) were attained when compared to those reached herein. According to Fickers et al. (2005), fatty acids synthesized after metabolization of some chain-numbered alkanes from 14 to 18 carbon atoms could be directly used for lipids synthesis and transported and stored in lipid bodies inside the yeast cells. This may explain the presence of an unusually high amount of palmitoleic acid (15 % – 30 %), since the HC used as a carbon source (hexadecane and hexadecene) may have been channeled directly to lipid bodies, resulting in lipids rich in fatty acids with a similar number of carbon atoms (16) as the HC assimilated by the yeasts. In Y. lipolytica CCY 30-26-36, the authors observed that a significant improvement in pentadecanoic acid (C15:0) was obtained in cultures using pentadecane as a carbon source, while in the presence of heptadecane, lipids produced had a high content of heptadecanoic acid (C17:0), probably due to metabolization of these alkanes into fatty acids precursors with unusual number of carbon atoms, such as propionyl-CoA (Matatkova et al. 2017).
Regardless of the pulse strategy, the lipids accumulated by Y. lipolytica CBS 2075 have a fatty acids composition (C16:0 and C18:1 > 30 %) similar to palm oil (C16:0–42 %; C18:0–4 %; C18:1–41 %; C18:2–10 %) (Giakoumis 2018). This particular fatty acid composition makes the lipids produced by Y. lipolytica CBS 2075 a good feedstock for biodiesel production and/or a promising substitute for palm oil (e.g., palm olein and palm stearin) employed in food products manufacturing (Ahmad et al. 2016), since dried and killed Y. lipolytica biomass cultivated in biofuel wastes have already been recognized as safe by the current food regulations (Jach and Malm 2022). On the other hand, lipids produced by the DSM 8218 strain had a low content in C18:1, which is an unusual outcome in Y. lipolytica cultures (Naveira-Pazos et al. 2023). Lipids produced by Y. lipolytica W29 from other hydrophobic substrates such as waste cooking oils (Lopes et al. 2019; Fabiszewska et al. 2022), pork lard (Lopes et al. 2018), and palm oil mill wastewater (Louhasakul et al. 2021) had a high content in C18:1 (26 % – 60%). Y. lipolytica DSM 8218 appears as a promising candidate to produce lipid-rich biomass with high content of palmitoleic acid (C16:1) (around 30 %), a valuable monounsaturated fatty acid rarely found in high amounts in microbial lipids and known for its broad applications in pharmaceutical and cosmetics industries (Kolouchová et al. 2015).
Pulse fed-batch was a suitable mode of operation for both lipids and protease production by these Y. lipolytica strains. The maximum protease activity reached in this work was similar to other values found in the literature for Y. lipolytica strains growing in castor oil (Gomes et al. 2013) and olive oil-based medium (Lopes et al. 2008), and even higher than those reported for Y. lipolytica W29 cultivated in waste cooking oils (Lopes et al. 2019) and pure glucose (Lopes et al. 2009). In hexadecane-based cultures, Y. lipolytica CBS 2075 achieved a maximum protease activity of approximately 500 U·L−1 (Miranda et al. 2024), suggesting that protease production in Y. lipolytica cultures from HC depends on the cultivation mode, HC concentration, and HC type. The substantial production of protease by Y. lipolytica utilizing an HC mixture represents a promising approach that aligns environmental sustainability with the production of high-value products. Proteases, being significant industrial biocatalysts, find diverse biotechnological applications, particularly in industries related to wastewater treatment (Naveed et al. 2021). The low values of lipolytic activity (< 40 U·L−1) reached may be due to the simultaneous secretion of high amounts of protease since these extracellular enzymes are responsible for the degradation of lipases (Najjar et al. 2011).
In the literature, it has been reported that cell-free samples of Y. lipolytica, after cultivation in heptadecane (Kim et al. 1999), used engine oil (Yalçın et al. 2018), and petroleum (Csutak et al. 2015) were able to emulsify different aliphatic and aromatic HC, as well as HC mixtures (e.g., crude oil), suggesting the secretion of biosurfactants by the yeast. A reduction in the surface tension of the medium significantly was observed in CBS 2075 and DSM 8218 supernatants (Table 5), possibly due to the production of biosurfactants by the yeasts, albeit in small amounts. Similar results of surface tension reduction (7 mN·m−1– 10 mN·m−1) were observed for Y. lipolytica IMUFRJ 50862 cultivated in crude oil (Ferreira et al. 2023). By contrast, Fontes et al. (2010) reported a lower reduction in the surface tension of the medium in the presence of hexadecane compared to glucose and glycerol. The authors suggested that Y. lipolytica IMUFRJ 50862 produced cell-bound biosurfactants rather than extracellular biosurfactants, as they observed the migration of Y. lipolytica cells from the aqueous phase (cultivation medium) to the organic phase (hexadecane).
Microbial adhesion to HC (MATH) is a parameter that provides insights into the alterations in microbial surface properties. As described by Rosenberg et al. (1980), microbial cell hydrophobicity is determined by the ability of cells to adhere to a hydrophobic surface, such as HC. Cells of Y. lipolytica CBS 2075 and DSM 8218 exhibited a high cell surface hydrophobicity after their growth in glucose (pre-inoculum), as occurred with other strains such as Y. lipolytica PG-20 and PG-32 in hydrocarbons (Hassanshahian et al. 2012), and Y. lipolytica NCIM 3589 in glucose and bromobenzene (Vatsal et al. 2017). In addition, Chrzanowski et al. (2008) emphasize the crucial role of the hydrophobic character of Y. lipolytica cells during yeast cultivation in HC. Their observations highlight that Y. lipolytica strains displaying high cell surface hydrophobicity (> 76 %) have enhanced ability in assimilating HC such as dodecane and hexadecane.
Conclusion
In summary, this study illustrates that Y. lipolytica can simultaneously assimilate higher alkanes (hexadecane and dodecane) and alkenes (hexadecene) while synthesizing valuable compounds. This represents an alternative approach for producing lipids-rich biomass and protease from pollutant compounds. In batch cultures, 30 % dissolved oxygen concentration was sufficient to achieve high yields of lipids and protease. Regardless of the Y. lipolytica strain, pulse fed-batch cultures improved the bioprocess performance. The addition of pulses of aliphatic hydrocarbons led to notable enhancements in biomass, lipids, and protease production compared to batch cultures. Lipids produced by Y. lipolytica CBS 2075 in pulse fed-batch cultures were rich in C16:0 and C18:1. Conversely, lipids from the DSM 8218 strain had a high content of C16:0, C16:1, and C18:1 with one pulse, while C16:0 and C16:1 were the main fatty acids with three pulses. Lipids produced by Y. lipolytica strains are a promising raw material for several industries, including food manufacturing, cosmetics, pharmaceuticals, and biodiesel. Regardless of the culture strategy studied, the presence of aliphatic hydrocarbons increased the cell surface hydrophobicity of Y. lipolytica. Furthermore, a reduction in the surface tension of the medium was observed, suggesting the production of extracellular biosurfactants, even at low amounts.
Data availability
No datasets were generated or analysed during the current study.
References
Ahmad FB, Zhang Z, Doherty WOS, O’Hara IM (2016) Evaluation of oil production from oil palm empty fruit bunch by oleaginous micro-organisms. Biofuels Bioprod Biorefin 10:378–392. https://doi.org/10.1002/bbb.1645
Alonso FOM, Oliveira EBL, Dellamora-Ortiz GM, Pereira-Meirelles FV (2005) Improvement of lipase production at different stirring speeds and oxygen levels. Braz J Chem Eng 22:9–18. https://doi.org/10.1590/S0104-66322005000100002
Amaral PFF, Lehocky M, Barros-Timmons AMV et al (2006) Cell surface characterization of Yarrowia lipolytica IMUFRJ 50682. Yeast 23:191–198. https://doi.org/10.1002/yea
Bellou S, Makri A, Triantaphyllidou IE et al (2014) Morphological and metabolic shifts of Yarrowia lipolytica induced by alteration of the dissolved oxygen concentration in the growth environment. Microbiology 160:807–817. https://doi.org/10.1099/mic.0.074302-0
Cafolla C, Voïtchovsky K (2020) Impact of water on the lubricating properties of hexadecane at the nanoscale. Nanoscale 12:14504–14513. https://doi.org/10.1039/d0nr03642k
Carsanba E, Papanikolaou S, Fickers P, Erten H (2020) Lipids by Yarrowia lipolytica strains cultivated on glucose in batch cultures. Microorganisms 8:1–14. https://doi.org/10.3390/microorganisms8071054
Castro AR, Guimarães M, Oliveira JV, Pereira MA (2017) Production of added value bacterial lipids through valorisation of hydrocarbon-contaminated cork waste. Sci Total Environ 605–606:677–682. https://doi.org/10.1016/j.scitotenv.2017.06.216
Cavallo E, Nobile M, Cerrutti P, Foresti ML (2020) Exploring the production of citric acid with Yarrowia lipolytica using corn wet milling products as alternative low-cost fermentation media. Biochem Eng J 155:107463. https://doi.org/10.1016/j.bej.2019.107463
Cerniglia CE, Crow SA (1981) Metabolism of aromatic hydrocarbons by yeasts. Arch Microbiol 129:9–13. https://doi.org/10.1007/BF00417170
Chrzanowski Ł, Bielicka-Daszkiewicz K, Owsianiak M et al (2008) Phenol and n-alkanes (C12 and C16) utilization: influence on yeast cell surface hydrophobicity. World J Microbiol Biotechnol 24:1943–1949. https://doi.org/10.1007/s11274-008-9704-8
Crolla A, Kennedy KJ (2004) Fed-batch production of citric acid by Candida lipolytica grown on n-paraffins. J Biotechnol 110:73–84. https://doi.org/10.1016/j.jbiotec.2004.01.007
Csutak O, Corbu V, Stoica I et al (2015) Biotechnological applications of Yarrowia lipolytica CMGB32. Agric Agric Sci Procedia 6:545–553. https://doi.org/10.1016/j.aaspro.2015.08.083
Darvishi F, Salmani N, Hosseini B (2019) Biovalorization of vegetable oil refinery wastewater into value-added compounds by Yarrowia lipolytica. J Chem Technol Biotechnol 94:2961–2968. https://doi.org/10.1002/jctb.6102
Deeba F, Pruthi V, Negi YS (2018) Aromatic hydrocarbon biodegradation activates neutral lipid biosynthesis in oleaginous yeast. Bioresour Technol 255:273–280. https://doi.org/10.1016/j.biortech.2018.01.096
Dias B, Fernandes H, Lopes M, Belo I (2023) Yarrowia lipolytica produces lipid-rich biomass in medium mimicking lignocellulosic biomass hydrolysate. Appl Microbiol Biotechnol 107:3925–3937. https://doi.org/10.1007/s00253-023-12565-6
Dobrowolski A, Drzymała K, Rzechonek DA et al (2019) Lipid production from waste materials in seawater-based medium by the yeast Yarrowia lipolytica. Front Microbiol 10:1–9. https://doi.org/10.3389/fmicb.2019.00547
Fabiszewska AU, Zieniuk B, Kozłowska M et al (2021) Studies on upgradation of waste fish oil to lipid-rich yeast biomass in Yarrowia lipolytica batch cultures. Foods 10:1–15. https://doi.org/10.3390/foods10020436
Fabiszewska A, Wierzchowska K, Nowak D et al (2022) Brine and post-frying oil management in the fish processing industry—a concept based on oleaginous yeast culture. Processes 10:1–12. https://doi.org/10.3390/pr10020294
Ferreira P, Lopes M, Mota M, Belo I (2016) Oxygen transfer rate and pH as major operating parameters of citric acid production from glycerol by Yarrowia lipolytica W29 and CBS 2073. Chem Pap 70:869–876. https://doi.org/10.1515/chempap-2016-0024
Ferreira TF, Martins FF, Cayres CA et al (2023) Biosurfactant production from the biodegradation of n-paraffins, isoprenoids and aromatic hydrocarbons from crude petroleum by Yarrowia lipolytica IMUFRJ 50682. Fermentation 9:21. https://doi.org/10.3390/fermentation9010021
Fickers P, Benetti PH, Waché Y et al (2005) Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res 5:527–543. https://doi.org/10.1016/j.femsyr.2004.09.004
Finogenova TV, Morgunov IG, Kamzolova SV, Cherniavskaia OG (2005) Organic acid production by the yeast Yarrowia lipolytica: a review of prospects. Appl Biochem Microbiol 41:478–486
Fontes GC, Amaral PFF, Nele M, Coelho MAZ (2010) Factorial design to optimize biosurfactant production by Yarrowia lipolytica. J Biomed Biotechnol. https://doi.org/10.1155/2010/821306
Fukuda R (2023) Utilization of n-alkane and roles of lipid transfer proteins in Yarrowia lipolytica. World J Microbiol Biotechnol 39:1–11. https://doi.org/10.1007/s11274-023-03541-3
Giakoumis EG (2018) Analysis of 22 vegetable oils’ physico-chemical properties and fatty acid composition on a statistical basis, and correlation with the degree of unsaturation. Renew Energy 126:403–419. https://doi.org/10.1016/j.renene.2018.03.057
Gomes N, Braga A, Teixeira JA, Belo I (2013) Impact of lipase-mediated hydrolysis of castor oil on γ-decalactone production by Yarrowia lipolytica. J Am Oil Chem Soc 90:1131–1137. https://doi.org/10.1007/s11746-013-2231-2
Gudiña EJ, Pereira JFB, Rodrigues LR et al (2012) Isolation and study of microorganisms from oil samples for application in Microbial Enhanced Oil Recovery. Int Biodeterior Biodegrad 68:56–64. https://doi.org/10.1016/j.ibiod.2012.01.001
Hassanshahian M, Tebyanian H, Cappello S (2012) Isolation and characterization of two crude oil-degrading yeast strains, Yarrowia lipolytica PG-20 and PG-32, from the Persian Gulf. Mar Pollut Bull 64:1386–1391. https://doi.org/10.1016/j.marpolbul.2012.04.020
Imron MF, Kurniawan SB, Ismail NI, Abdullah SRS (2020) Future challenges in diesel biodegradation by bacteria isolates: a review. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.119716
Jach ME, Malm A (2022) Yarrowia lipolytica as an alternative and valuable source of nutritional and bioactive compounds for humans. Molecules 27:27–29. https://doi.org/10.3390/molecules27072300
Janek T, Mirończuk AM, Rymowicz W, Dobrowolski A (2020) High-yield expression of extracellular lipase from Yarrowia lipolytica and its interactions with lipopeptide biosurfactants: a biophysical approach. Arch Biochem Biophys. https://doi.org/10.1016/j.abb.2020.108475
Kar T, Destain J, Thonart P, Delvigne F (2010) Impact of scaled-down on dissolved oxygen fluctuations at different levels of the lipase synthesis pathway of Yarrowia lipolytica. Biotechnol Agron Soc Environ 14:523–529
Kebede G, Tafese T, Abda EM et al (2021) Factors influencing the bacterial bioremediation of hydrocarbon contaminants in the soil: mechanisms and impacts. J Chem. https://doi.org/10.1155/2021/9823362
Kim T-H, Lee J-H, Oh Y-S et al (1999) Identification and characterization of an oil-degrading. J Microbiol 37:128–135
Kolouchová I, Sigler K, Schreiberová O et al (2015) New yeast-based approaches in production of palmitoleic acid. Bioresour Technol 192:726–734. https://doi.org/10.1016/j.biortech.2015.06.048
Krummenacher JJ, West KN, Schmidt LD (2003) Catalytic partial oxidation of higher hydrocarbons at millisecond contact times: decane, hexadecane, and diesel fuel. J Catal 215:332–343. https://doi.org/10.1016/S0021-9517(03)00011-3
Kuppusamy S, Maddela NR, Megharaj M, Venkateswarlu K (2020) An overview of total petroleum hydrocarbons. In: total petroleum hydrocarbons: environmental fate, toxicity, and remediation. Springer International Publishing, Cham, pp 1–27
Ławniczak Ł, Woźniak-Karczewska M, Loibner AP et al (2020) Microbial degradation of hydrocarbons—basic principles for bioremediation: a review. Molecules 25:1–19. https://doi.org/10.3390/molecules25040856
Lazar Z, Dulermo T, Neuvéglise C et al (2014) Hexokinase-A limiting factor in lipid production from fructose in Yarrowia lipolytica. Metab Eng 26:89–99. https://doi.org/10.1016/j.ymben.2014.09.008
Llamas M, Dourou M, González-Fernández C et al (2020) Screening of oleaginous yeasts for lipid production using volatile fatty acids as substrate. Biomass Bioenerg 138:1–10. https://doi.org/10.1016/j.biombioe.2020.105553
Lopes M, Gomes N, Gonçalves C et al (2008) Yarrowia lipolytica lipase production enhanced by increased air pressure. Lett Appl Microbiol 46:255–260. https://doi.org/10.1111/j.1472-765X.2007.02299.x
Lopes M, Gomes N, Mota M, Belo I (2009) Yarrowia lipolytica growth under increased air pressure: influence on enzyme production. Appl Biochem Biotechnol 159:46–53. https://doi.org/10.1007/s12010-008-8359-0
Lopes M, Gomes AS, Silva CM, Belo I (2018) Microbial lipids and added value metabolites production by Yarrowia lipolytica from pork lard. J Biotechnol 265:76–85. https://doi.org/10.1016/j.jbiotec.2017.11.007
Lopes M, Miranda SM, Alves JM et al (2019) Waste cooking oils as feedstock for lipase and lipid-rich biomass production. Eur J Lipid Sci Technol 121:1–9. https://doi.org/10.1002/ejlt.201800188
Lopes M, Miranda SM, Costa AR et al (2022) Yarrowia lipolytica as a biorefinery platform for effluents and solid wastes valorization–challenges and opportunities. Crit Rev Biotechnol 42:163–183. https://doi.org/10.1080/07388551.2021.1931016
Louhasakul Y, Cheirsilp B (2013) Industrial waste utilization for low-cost production of raw material oil through microbial fermentation. Appl Biochem Biotechnol 169:110–122. https://doi.org/10.1007/s12010-012-9965-4
Louhasakul Y, Treu L, Kougias PG et al (2021) Valorization of palm oil mill wastewater for integrated production of microbial oil and biogas in a biorefinery approach. J Clean Prod 296:126606. https://doi.org/10.1016/j.jclepro.2021.126606
Magdouli S, Brar SK, Blais JF (2018) Morphology and rheological behaviour of Yarrowia lipolytica: impact of dissolved oxygen level on cell growth and lipid composition. Process Biochem 65:1–10. https://doi.org/10.1016/j.procbio.2017.10.021
Mao Y, Raza M, Wu Z et al (2020) An experimental study of n-dodecane and the development of an improved kinetic model. Combust Flame 212:388–402. https://doi.org/10.1016/j.combustflame.2019.11.014
Matatkova O, Gharwalova L, Zimola M et al (2017) Using odd-alkanes as a carbon source to increase the content of nutritionally important fatty acids in Candida krusei, Trichosporon cutaneum, and Yarrowia lipolytica. Int J Anal Chem. https://doi.org/10.1155/2017/8195329
Miranda SM, Lopes M, Belo I (2024) Exploring the use of hexadecane by Yarrowia lipolytica: effect of dissolved oxygen and medium supplementation. J Biotechnol 380:29–37. https://doi.org/10.1016/j.jbiotec.2023.12.006
Morales-Palomo S, González-Fernández C, Tomás-Pejó E (2022) Prevailing acid determines the efficiency of oleaginous fermentation from volatile fatty acids. J Environ Chem Eng 10:107354. https://doi.org/10.1016/j.jece.2022.107354
Najjar A, Robert S, Guérin C et al (2011) Quantitative study of lipase secretion, extracellular lipolysis, and lipid storage in the yeast Yarrowia lipolytica grown in the presence of olive oil: analogies with lipolysis in humans. Appl Microbiol Biotechnol 89:1947–1962. https://doi.org/10.1007/s00253-010-2993-5
Naveed M, Nadeem F, Mehmood T et al (2021) Protease—a versatile and ecofriendly biocatalyst with multi-industrial applications: an updated review. Catal Lett 151:307–323. https://doi.org/10.1007/s10562-020-03316-7
Naveira-Pazos C, Robles-Iglesias R, Fernández-Blanco C et al (2023) State-of-the-art in the accumulation of lipids and other bioproducts from sustainable sources by Yarrowia lipolytica. Rev Environ Sci Bio/Technol 22:1131–1158. https://doi.org/10.1007/s11157-023-09670-3
Noveiri H, Nozari M, Almasi H et al (2023) Biodegradation of N-hexadecane using bacterial consortium isolated from seawater contaminated with petroleum. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-023-04115-x
Papanikolaou S, Aggelis G (2010) Yarrowia lipolytica: a model microorganism used for the production of tailor-made lipids. Eur J Lipid Sci Technol 112:639–654. https://doi.org/10.1002/ejlt.200900197
Papanikolaou S, Chevalot I, Komaitis M et al (2002) Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Appl Microbiol Biotechnol 58:308–312. https://doi.org/10.1007/s00253-001-0897-0
Papanikolaou S, Galiotou-Panayotou M, Chevalot I et al (2006) Influence of glucose and saturated free-fatty acid mixtures on citric acid and lipid production by Yarrowia lipolytica. Curr Microbiol 52:134–142. https://doi.org/10.1007/s00284-005-0223-7
Papanikolaou S, Chevalot I, Galiotou-Panayotou M et al (2007) Industrial derivative of tallow: a promising renewable substrate for microbial lipid, single-cell protein and lipase production by Yarrowia lipolytica. J Biotechnol 10:425–435. https://doi.org/10.2225/vol10-issue3-fulltext-8
Papanikolaou S, Diamantopoulou P, Blanchard F et al (2020) Physiological characterization of a novel wild-type Yarrowia lipolytica strain grown on glycerol: effects of cultivation conditions and mode on polyols and citric acid production. Appl Sci 10:1–24. https://doi.org/10.3390/app10207373
Pereira AS, Miranda SM, Lopes M, Belo I (2021) Factors affecting microbial lipids production by Yarrowia lipolytica strains from volatile fatty acids: effect of co-substrates, operation mode and oxygen. J Biotechnol 331:37–47. https://doi.org/10.1016/j.jbiotec.2021.02.014
Pereira AS, Lopes M, Miranda SM, Belo I (2022) Bio-oil production for biodiesel industry by Yarrowia lipolytica from volatile fatty acids in two-stage batch culture. Appl Microbiol Biotechnol 106:2869–2881. https://doi.org/10.1007/s00253-022-11900-7
Rakicka M, Lazar Z, Dulermo T et al (2015) Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol Biofuels 8:104. https://doi.org/10.1186/s13068-015-0286-z
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33. https://doi.org/10.1111/j.1574-6968.1980.tb05599.x
Rywińska A, Tomaszewska-Hetman L, Juszczyk P et al (2024) Enhanced production of erythritol from glucose by the newly obtained UV mutant Yarrowia lipolytica K1UV15. Molecules. https://doi.org/10.3390/molecules29102187
SadrAzodi SM, Shavandi M, Amoozegar MA, Mehrnia MR (2019) Biodegradation of long chain alkanes in halophilic conditions by Alcanivorax sp. strain Est-02 isolated from saline soil. 3 Biotech 9:1–10. https://doi.org/10.1007/s13205-019-1670-3
Sarris D, Rapti A, Papafotis N et al (2019) Production of added-value chemical compounds through bioconversions of olive-mill wastewaters blended with crude glycerol by a Yarrowia lipolytica strain. Molecules 24:1–26. https://doi.org/10.3390/molecules24020222
Snopek P, Nowak D, Zieniuk B, Fabiszewska A (2021) Aeration and stirring in Yarrowia lipolytica lipase biosynthesis during batch cultures with waste fish oil as a carbon source. Fermentation 7:1–11. https://doi.org/10.3390/fermentation7020088
Tang X, Kaskiewicz PL, Camacho Corzo DM et al (2018) Solubility and crystallisability of the ternary system: hexadecane and octadecane representative in fuel solvents. Fuel 226:665–674. https://doi.org/10.1016/j.fuel.2018.04.022
Thorat BN, Sonwani RK (2022) Current technologies and future perspectives for the treatment of complex petroleum refinery wastewater: a review. Bioresour Technol 355:127263. https://doi.org/10.1016/j.biortech.2022.127263
Tomaszewska L, Rakicka M, Rymowicz W, Rywińska A (2014) A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res 14:966–976. https://doi.org/10.1111/1567-1364.12184
Tomaszewska-Hetman L, Rywińska A, Lazar Z et al (2021) Application of a new engineered strain of Yarrowia lipolytica for effective production of calcium ketoglutarate dietary supplements. Int J Mol Sci. https://doi.org/10.3390/ijms22147577
Tzintzun-Camacho O, Loera O, Ramírez-Saad HC, Gutiérrez-Rojas M (2012) Comparison of mechanisms of hexadecane uptake among pure and mixed cultures derived from a bacterial consortium. Int Biodeterior Biodegrad 70:1–7. https://doi.org/10.1016/j.ibiod.2012.01.009
Vasiliadou IA, Bellou S, Daskalaki A et al (2018) Biomodification of fats and oils and scenarios of adding value on renewable fatty materials through microbial fermentations: modelling and trials with Yarrowia lipolytica. J Clean Prod 200:1111–1129. https://doi.org/10.1016/j.jclepro.2018.07.187
Vatsal AA, Zinjarde SS, RaviKumar A (2017) Phenol is the initial product formed during growth and degradation of bromobenzene by tropical marine yeast, Yarrowia lipolytica NCIM 3589 via an early dehalogenation step. Front Microbiol 8:1–15. https://doi.org/10.3389/fmicb.2017.01165
Yalçın HT, Ergin-Tepebaşı G, Uyar E (2018) Isolation and molecular characterization of biosurfactant producing yeasts from the soil samples contaminated with petroleum derivatives. J Basic Microbiol 58:782–792. https://doi.org/10.1002/jobm.201800126
Yang L, Xu X, Yan J et al (2024) Linking metabolomics to machine learning reveals the metabolic fates of the refractory industrial pollutant 1-hexadecene. Chem Eng J 488:150920. https://doi.org/10.1016/j.cej.2024.150920
Zhong H, Liu Y, Liu Z et al (2014) Degradation of pseudo-solubilized and mass hexadecane by a Pseudomonas aeruginosa with treatment of rhamnolipid biosurfactant. Int Biodeterior Biodegrad 94:152–159. https://doi.org/10.1016/j.ibiod.2014.07.012
Funding
Open access funding provided by FCT|FCCN (b-on). This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020 unit and doctoral grant (SFRH/BD/144188/2019).
Author information
Authors and Affiliations
Contributions
Sílvia Miranda: investigation, methodology, formal analysis, methodology, writing—original draft. Isabel Belo: supervision, conceptualization, writing—review & editing. Marlene Lopes: supervision, conceptualization, writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Research involving human and/or animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miranda, S.M., Belo, I. & Lopes, M. Yarrowia lipolytica growth, lipids, and protease production in medium with higher alkanes and alkenes. World J Microbiol Biotechnol 40, 318 (2024). https://doi.org/10.1007/s11274-024-04123-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-024-04123-7