Abstract
Bacterial extracellular vesicles (BEVs) are non-replicative nanostructures released by Gram-negative and Gram-positive bacteria as a survival mechanism and inter- and intraspecific communication mechanism. Due to BEVs physical, biochemical, and biofunctional characteristics, there is interest in producing and using them in developing new therapeutics, vaccines, or delivery systems. However, BEV release is typically low, limiting their application. Here, we provide a biotechnological perspective to enhance BEV production, highlighting current strategies. The strategies include the production of hypervesiculating strains through gene modification, bacteria culture under stress conditions, and artificial vesicles production. We discussed the effect of these production strategies on BEVs types, morphology, composition, and activity. Furthermore, we summarized general aspects of BEV biogenesis, functional capabilities, and applications, framing their current importance and the need to produce them in abundance. This review will expand the knowledge about the range of strategies associated with BEV bioprocesses to increase their productivity and extend their application possibilities.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular vesicles (EVs) are non-replicative membranous nanostructures released into the extracellular environment by eukaryotic cells, archaea, and bacteria (Bitto and Kaparakis-Liaskos 2017; Gill et al. 2019; Joffe et al. 2016). Particularly, Gram-negative, and Gram-positive bacteria produce extracellular vesicles (BEVs) during all phases of growth and in various environments (Bitto and Kaparakis-Liaskos 2017; Kim et al. 2015a; Klimentova et al. 2019; Koning et al. 2013; Schwechheimer and Kuehn 2015; Wang et al. 2021a, b). The release of BEVs is a conserved process recognized as a “type zero secretion system” (Bitto and Kaparakis-Liaskos 2017; Guerrero-Mandujano et al. 2017; Mozaheb and Mingeot-Leclercq 2020).
Most BEVs are spherical particles with heterogeneous sizes ranging from 10 to 400 nm in diameter (Bitto and Kaparakis-Liaskos 2017; Toyofuku et al. 2019; Uddin et al. 2020). Due to the differences in the cell wall between Gram-negative and Gram-positive bacteria, the biogenesis and composition of BEVs are different (Brown et al. 2015; Kim et al. 2015a; Toyofuku et al. 2019; Yáñez-Mó et al. 2015). Gram-negative bacteria usually release BEVs from the outer membrane (OM), called OMVs (Outer Membrane Vesicles) (Hu et al. 2020; Schwechheimer and Kuehn 2015), although the formation of outer-inner membrane vesicles (O-IMVs) has also been described (Gill et al. 2019; Pérez-Cruz et al. 2015; Toyofuku et al. 2019). OMVs and O-IMVs are composed of lipopolysaccharides (LPS), membrane lipids, peptidoglycan (PG), outer membrane proteins (OMPs), periplasmic proteins, and metabolites (Gill et al. 2019; Jan 2017; Pérez-Cruz et al. 2015; Thoma et al. 2018; Uddin et al. 2020). O-IMVs are additionally enriched for cytoplasmic (including nucleic acids) and inner membrane (IM) components when compared to OMVs (Gill et al. 2019). In contrast, Gram-positive bacteria release vesicles from the cell membrane (CM), called membrane vesicles (MV) (Cao and Lin 2021; Toyofuku et al. 2019), carrying as cargo nucleic acids, membrane and cytoplasmic proteins, membrane lipids, lipoteichoic acids (LTA), and other metabolites (Brown et al. 2015; Cao and Lin 2021).
BEVs play essential roles in bacterial survival, cell communication, infection, or cell–cell interaction (Caruana and Walper 2020; Liu et al. 2018; Schwechheimer et al. 2015; Zlatkov et al. 2021). Bacteria use BEVs to send information via effector molecules to target cells (Bitto and Kaparakis-Liaskos 2017; Gill et al. 2019; Yáñez-Mó et al. 2015), and depending on the recipient cell, cargo molecules can be delivered through membrane fusion or by endocytosis (Bitto et al. 2017; Cañas et al. 2016; Ñahui et al. 2021; Wolf et al. 2012; Yáñez-Mó et al. 2015). Furthermore, the delivery to specific cells could be directed by molecules located on the external surface of BEVs (Kaparakis-Liaskos and Ferrero 2015; Yáñez-Mó et al. 2015). While the lipid bilayer protects the cargo from adverse environmental conditions or degradative enzymes, allowing the transport of information (Bonnington and Kuehn 2014; Caruana and Walper 2020; Gill et al. 2019; Guerrero-Mandujano et al. 2017; Peng et al. 2020).
Due to BEV composition and ability to transport different cargo and information, their use in developing therapeutics, vaccines, and drug delivery systems has become a relevant research topic (Huang et al. 2022a; Liu et al. 2022b,c,d; Thomas et al. 2022). However, different limitations have been identified in the commercial use of BEVs, highlighting among them the low production yields (García-Manrique et al. 2018; Hahm et al. 2021; Hu et al. 2020; Morishita et al. 2021).
Different reviews explain key aspects of the biology of BEVs, as well as some research on genetic engineering, physical, chemical, and biotechnological strategies to bioengineer BEVs and/or increase their production (Brown et al. 2015; García-Manrique et al. 2018; Gnopo et al. 2017; Liu et al. 2022b,d; Richter et al. 2021a; Schwechheimer et al. 2015; Toyofuku et al. 2019). This review compiles relevant biological aspects of BEVs, covering information on biogenesis mechanisms, known biofunctions, and recent applications in developing new biotherapeutics. We discuss in detail strategies to produce and increase the release of BEVs and the effects of these strategies on the morphology, composition, and activity of the resulting structures to improve knowledge for its feasible biotechnological application, considering the need for its production in large quantities. The production strategies were divided into three categories: molecular modifications of strains, cultivation under stress conditions, and production and recovery of artificial BEVs. Therefore, the understanding of BEVs and integration of available strategies for bioprocess development focused on the abundant production of BEVs, will improve their productivity and biotechnological application.
Biogenesis mechanisms of BEVs
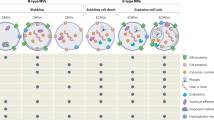
The biogenesis of BEVs depends on the composition of the cell wall or type of bacteria. Therefore, BEV formation mechanisms between Gram-negative and Gram-positive bacteria differ from each other, the formation of the latter being the least characterized (Briaud and Carroll 2020; Nagakubo et al. 2020; Ñahui et al. 2021). Currently, multiple mechanisms are reported to explain the generation of BEVs (Schwechheimer et al. 2013, 2014; Pathirana and Kaparakis-Liaskos 2016) divided at least into three models, not mutually exclusive: blebbing or budding of CM (Fig. 1A,C), explosive cell lysis or bubbling cell death (Fig. 1B, D), and formation of nanotubes (Fig. 1E) (Jeong et al. 2022; Gill et al. 2019; Toyofuku et al. 2019).
Biogenesis mechanisms of Bacterial Extracellular Vesicles (BEVs) models: (A) Blebbing and B) explosive cell lysis in Gram-negative bacteria, (C) Budding of the cellular membrane (CM) and (D) bubbling cell death in Gram-positive bacteria, and (E) nanotube formation in both. Blebbing and budding can be caused by factors such as loss of cross-links between peptidoglycan (PG) and outer membrane (OM), changes in the membrane composition, molecules intercalation like with Pseudomonas Quinolone Signals (PQS), or turgor pressure due to molecule accumulation in the cell wall, i.e., LPS and PG fragments, proteins, or Phenol-soluble modulins (PSMs). The explosive cell lysis and bubbling cell death are attributed to endolysins. Endolysins degrade the PG, damaging cells. Nanotubes are generated by the extrusion of the cellular membrane (CM) in Gram-positive bacteria and OM in Gram-negative bacteria. Created with Servier Medical Art resources
Blebbing and budding of cell membranes
In Gram-negative bacteria, blebbing occurs when the OM dissociates from the PG, protrudes to the outside, closes on itself, and detaches from the cell envelope (Schwechheimer and Kuehn 2015; Toyofuku et al. 2019). Blebbing is the main model of OMVs formation (Nagakubo et al. 2020; Toyofuku et al. 2019; Zingl et al. 2020), is triggered by three factors: (i) the modulation of the cross-links in the envelope (OM–PG), (ii) the modification in the OM composition, and (iii) the turgor stress (Fig. 1A) (Bernadac et al. 1998; Hayashi et al. 2002; McBroom et al. 2006; Schwechheimer et al. 2014; Toyofuku et al. 2019; Zingl et al. 2020).
The cross-links in the envelope have been associated with vesicular biogenesis due to the role they play in the union and, in the integrity of the cell envelope in Gram-negative bacteria. Particularly, genes coding for the membrane porin OmpA in Salmonella, the lipoprotein NlpI and Tol-Pal system proteins in Escherichia coli, have been associated with vesicular biogenesis (Deatherage et al. 2009; Nevermann et al. 2019; Schwechheimer et al. 2014, 2015; Schwechheimer and Kuehn 2013). Disruptions in these genes prevent the formation of junctions between PG and OM, leading to the shedding of the OM in the form of OMVs (Deatherage et al. 2009; Schwechheimer and Kuehn 2015). In the same sense, the rearrangement or abundance of OM-PG bonds is regulated by small RNAs (sRNA), such as Reg26 and MicA, related to the down-expression of lipoproteins (Lpps) and OmpA, respectively (Choi et al. 2017; Schwechheimer et al. 2013; Song et al. 2008; Zingl et al. 2020).
The modification of components of the OM, such as the accumulation of phospholipids in Haemophilus influenza and Vibrio cholerae, or the introduction of molecules, such as the quinolone signal in Pseudomonas aeruginosa (Pseudomonas Quinolone Signal), alter the curvature of the membrane, promoting the release of OMVs (Florez et al. 2017; Gill et al. 2019; Roier et al. 2016; Toyofuku et al. 2019). Likewise, the structure of LPS, such as the deacylation of lipid A in Salmonella typhimurium, generates a differential curvature that results in the generation of OMVs (Elhenawy et al. 2016; Mozaheb and Mingeot-Leclercq 2020).
Turgor stress is the third factor related to the formation of OMVs by blebbing, arising from the accumulation of misfolded proteins, PG fragments, or LPS in the periplasm of Gram-negative bacteria (Ojima et al. 2016; Schwechheimer et al. 2014; Schwechheimer and Kuehn 2013). The abundant presence of these molecules increases the pressure on the cell wall, with the generation of OMVs being the mechanism to release intracellular stress to maintain cell homeostasis (Gill et al. 2019; Schwechheimer et al. 2014; Toyofuku et al. 2019).
In Gram-positive bacteria, BEV biogenesis can start with the budding or blebbing of CM (Fig. 1C) (Briaud and Carroll 2020; Jeong et al 2022). Lipidomic analyses of MVs have shown differences in phospholipid and fatty acid content between the vesicles and the CM (Briaud and Carroll 2020). MVs of Listeria monocytogenes present an abundance of phosphatidylethanolamine, triacylglycerols, and sphingolipids (Coelho et al. 2019), while MVs derived from Lactobacillus plantarum are abundant in phosphatidylcholine, diacylglycerol and lysophosphatidylserine (Kim et al. 2020a). This differential composition of lipids between vesicles and cells suggests that the production of MVs by Gram-positive bacteria could be linked to lipid domains enriched in the CM (Briaud and Carroll 2020).
In Staphylococcus aureus, the biogenesis of MVs can also be promoted by phenol-soluble modulins (PSMs) (Wang et al. 2018). The PSMs correspond to a family of amphipathic peptides with surfactant activity that cause local deformations in the CM associated with an increase in the turgor pressure of the cytoplasm, increases the curvature of the membrane, which detaches forming new MVs (Wang et al. 2020, 2018). The participation of Lpps in MV biogenesis in S. aureus has also been suggested (Wang et al. 2020).
The MVs outflow through the PG layer of Gram-positive bacteria has been related to PG-degrading enzymes or autolysins, observed in S. aureus or Mycobacterium tuberculosis (Briaud and Carroll 2020; Lee et al. 2009; Palacios et al. 2020; Prados-Rosales et al. 2011; Wang et al. 2021a, b). Additionally, the degree of links in the PG affects MV production (Briaud and Carroll 2020), as has been observed with S. aureus subjected to sublethal concentrations of penicillin G that decreases PG binding and increases MV release without affecting cell viability (Wang et al. 2018; Wyke et al. 1981).
Explosive cell lysis and bubbling cell death
Cell lysis can lead to the biogenesis of diverse types of vesicles, OMVs, non-classical OMVs, or MVs, from Gram-negative membranes (Turnbull et al. 2016). Explosive cell lysis is triggered by the action of endolysins encoded in cryptic prophages, which are activated by DNA damage, and their role is to degrade PG in P. aeruginosa (Toyofuku et al. 2019; Turnbull et al. 2016). The BEV production mediated by endolysins in Gram-positive bacteria such as Bacillus subtilis and Lactococcus lactis has also been studied (Toyofuku et al. 2017; Liu et al. 2022a). In these bacteria, phage-encoded enzymes generate holes in the cell wall, allowing the release of the MVs, and although there is no explosive cell lysis, the cells die due to loss of cell integrity through the formation of bubbles, a process called bubbling cell death (Briaud and Carroll 2020; Toyofuku et al. 2017).
Formation of nanotubes in the production of BEVs
The formation of filamentous structures called nanotubes has been found in a wide variety of bacteria, including Gram-positives (B. subtilis and Clostridium acetobutylicum) and Gram-negatives (E. coli and Acinetobacter baylyi) (Baidya et al. 2018). The simplest morphology of the nanotubes is related to chains of OMVs, observed for the first time in cultures of Myxococcus xanthus (Remis et al. 2014; Toyofuku et al. 2019). These nanotubes participate in cell–cell communication and are considered a type of specialized BEVs (Baidya et al. 2018; Gill et al. 2019; Toyofuku et al. 2019). In Gram-positive bacteria, nanotube formation occurs through a process of extrusion of the plasma membrane through holes in the PG, while in Gram-negative bacteria, nanotubes are formed from the extrusion of the OM (Gill et al. 2019; Toyofuku et al. 2019) (Fig. 1).
Biological functions of BEVs
BEVs determine physiological functions in the life of the cells that produce them, despite their energetic or metabolic cost (Schwechheimer and Kuehn 2015) (Fig. 2).
Biological functions of Bacterial Extracellular Vesicles (BEVs): bacterial survival, bacterium-bacterium communication, and bacterium-host communication. BEVs may act like decoys and release key enzymes involved in activities such as substrate degradation, metal acquisition, or antibiotic resistance. BEVs also help the cell release pressure. BEVs play beneficial or antagonistic roles during the communication process with other cells. They can transfer genes, enzymes, molecules to activate quorum sensing, and substrates to other bacteria. Also, BEVs could carry virulence factors to promote invasion by pathogenic bacteria
BEVs in the bacterial survival
In natural and artificial environments, bacteria are exposed to unfavorable biotic and/or abiotic factors, which affect their growth and viability (Guan et al. 2017; Mozaheb and Mingeot-Leclercq 2020). Whereas BEVs participate in molecular mechanisms that ensure the survival of bacteria, increasing their production (Guerrero-Mandujano et al. 2017; McBroom et al. 2006).
BEVs released by Pseudomonas putida, E. coli, or V. cholerae have been found to function as decoys in the presence of toxic or antimicrobial compounds such as toluene, polymyxin B, and colistin (Giacomucci et al. 2022; Kobayashi et al. 2000; Manning and Kuehn 2011). Another bacterial survival strategy against harmful compounds is loading vesicles with hydrolytic enzymes, such as β-lactamase in BEVs of S. aureus and Moraxella catarrhalis, conferring resistance to ampicillin and amoxicillin, respectively (Lee et al. 2013; Schaar et al. 2011). Bacteria like Delftia acidovorans and Fibrobacter succinogenes release OMVs and nanotubes, loaded with enzymes involved in the degradation of unconventional carbon sources such as phenanthrene, hemicellulose, and pectin, favoring bacterial survival in complex media (Arntzen et al. 2017; Shetty and Hickey 2014). Another function of BEVs is the acquisition of metallic elements, such as iron and zinc, essential for the survival of Neisseria meningitidis (Lappann et al. 2013; Schwechheimer and Kuehn 2015).
BEVs in bacterium-bacterium interactions
BEVs are also used by bacteria as a communication mechanism with other bacteria, making these cell–cell interactions beneficial or antagonistic (Caruana and Walper 2020; Mozaheb and Mingeot-Leclercq 2020). In bacterium-bacterium communication, BEVs might increase genetic diversity and modify the survival of other bacteria through horizontal gene transfer (Dell’Annunziata et al. 2021; Goreham et al. 2019; Kim et al. 2015a; Klieve et al. 2005; Schwechheimer and Kuehn 2015). Furthermore, BEVs disperse signals during quorum sensing in P. aeruginosa cultures (Caruana and Walper 2020; Lee et al. 2009; Lin et al. 2017) or participate as nucleation sites and in the transport of compounds during biofilms formation by Streptococcus mutans and Shewanella vesiculosa (Baeza and Mercade 2020; Begić and Josić 2020; Caruana and Walper 2020; Wang et al. 2015; Liao et al. 2014). BEVs released in a microbial community, generate other benefits, such as access to nutrients. Bacteroides fragilis and Bacteroides thetaiotaomicron release BEVs loaded with hydrolytic proteins, leads to the generation of molecules that are easily assimilated by other species of the same community (Caruana and Walper 2020; Carvalho et al. 2019; Elhenawy et al. 2014). Among the antagonistic functions of BEVs, the release of virulence factors and antimicrobial compounds are observed, affecting the viability of other bacteria (Caruana and Walper 2020; Tashiro et al. 2013). Outstanding examples are the inhibition of E. coli by BEVs of Cytobacter velatus, or the viability reduction of Lactobacillus delbrueckii by bacteriocin-loaded Lactobacillus acidophilus MVs (Dean et al. 2020; Schulz et al. 2018).
BEVs in bacterium-host interactions
The study of communication between bacteria and eukaryotic cells through BEVs is another field of interest due to its influence on multiple diseases (Pathirana and Kaparakis-Liaskos 2016; Caruana and Walper 2020). Both, commensal and pathogenic bacteria can deliver effector molecules to mammalian cells, triggering cytotoxic, cytolytic, or immunomodulatory responses (Kim et al. 2015a; Pathirana and Kaparakis-Liaskos 2016). The type of response will depend on the origin of the BEVs (bacterial species), their concentration, and the target cell (Gurung et al. 2011; Kim et al. 2015a; Wang et al. 2021a, b). Generally, adverse responses are generated by vesicles of pathogenic bacteria (E. coli, Shigella dysenteriae, V. cholerae, P. aeruginosa and S. aureus), loaded with virulence factors, and their functions may correspond to invasion, adherence, damage to host cells or induction, of proinflammatory processes (Hu et al. 2020; Li et al. 2020; Wang et al. 2021a, b; Guerrero-Mandujano et al. 2017; Gurung et al. 2011). Meanwhile, BEVs from probiotic bacteria like Lactobacillus sp or E. coli Nissle 1917 carry effectors that protect the integrity of the intestinal epithelium against infections, maintain colorectal homeostasis during inflammation, and attenuate colitis or intestinal inflammation (Alvarez et al. 2019; Choi et al. 2020). In addition, BEVs of Bacteroides thetaiotaomicron transport enzymes that provide nutrients to the bacterium and remove carcinogenic metabolites from the host intestine (Carvalho et al. 2019; Goreham et al. 2019; Stentz et al. 2014). In plants, the activation of immune responses in the presence of BEVs from Pseudomonas syringae and Pseudomonas fluorescens protects them from pathogenic microorganisms such as other bacteria and oomycetes (McMillan et al. 2021).
Biotechnological applications of BEVs and aspects to be resolved
Biotechnological applications of BEVs have gained attention because of their interesting activities, such as containing bacterial antigens, pathogen-associated molecular patterns (PAMPs), intracellular communication cargo proteins, and immune system modulators (Huang et al. 2022a, 2022b; Li et al. 2020; Thoma et al. 2018). Among the biotechnological applications of BEVs (Table 1, Fig. 3), vaccine agents (van der Pol et al. 2015; Micoli and MacLennan 2020) and drug delivery systems have the greatest interest (Herrmann et al. 2021; Li et al. 2020).
Bacterial Extracellular Vesicles (BEVs) applications. BEVs are biotherapeutics in the treatment of cancer, depression, or organ inflammation. BEVs as molecule transport systems. BEVs in the production of vaccines and antimicrobial compounds. BEVs as biomarkers, like tuberculosis screening. BEVs as a biotechnology tool for the study of lipid membrane permeability and membrane proteins. BEVs as nanoreactors in bioremediation or substrates enzymatic transformation. Created with Biorender.com
The treatment of N. meningitidis, with VA-MENGOC-BC, MenBvac, and MeNZB, with a least 70% efficacy against meningitis caused by MenB (Holst et al. 2013), and the approval of BEXSERO vaccine points to the importance of the production of vesicles and the compounds they can transport (Holst et al. 2013). The advantages of BEVs are that they are non-replicative entities that mimic a part of a pathogen without causing disease (Ellis and Kuehn 2010). BEVs sizes allow their biodistribution through lymphatic vessels, as well as their uptake by different cells (Ellis and Kuehn 2010). Although vesicle components and size are variable, they are molecular entities that allow the presentation of antigens to the presenting reinforcement cells, and they have adjuvant properties that stimulate immune responses (Ellis and Kuehn 2010; Ellis et al. 2010; van der Pol et al. 2015). Furthermore, some BEVs are stable around 4–40 °C and against chemical treatments (Arigita et al. 2004). Even more, the polarization of the membranes can provide vesicle protector properties. Whereas LPS contained in OMV might act as an activator of immune cells, such as monocytes/macrophages, through TLR2 or TLR4/MD2 that could activate NF-κB and IRF3 expression and other proinflammatory molecules, which can also lead to high reactogenicity of the vaccines (Kovacs et al. 2011; Maisonneuve et al. 2014; Mancini et al. 2020). Other important molecules in BEVs are capsular polysaccharides (CPS) and LPS. CPS, combined with carrier proteins, might produce a good vaccine antigen (Middelton et al., 2017). Therefore, BEVs have been coupled with different antigens and glycoantigens to trigger immunological memory (Mancini et al. 2020).
Interestingly, the bioengineering of BEVs is a field that has broadened its spectrum of application, allowing for a selection of the charge and specific target and therefore the rational design of new therapies (Bitto and Kaparakis-Liaskos 2017; García-Manrique et al. 2018; Herrmann et al. 2021). It is highlighted that BEVs can be loaded with exogenous molecules such as antigens, ligands, enzymes, therapeutic proteins, nucleic acids molecules, among others, through genetic engineering, physical or chemical processes (Alves et al. 2018; Carvalho et al. 2019; Peng et al. 2020). Among bioengineered BEVs, the Generalized Modules for Membrane Antigens (GMMA) have been designed as delivery tools that can serve as carriers of polysaccharides or heterologous protein antigens (Mancini et al. 2021; Micoli et al. 2021). The GMMA are OM vesicles obtained from gram-negative bacteria genetically modified to eliminate endotoxins, which could cause reactogenicity in humans, and to obtain an over-vesiculating phenotype that improves yields (Di Benedetto et al. 2021; Hu et al. 2022; Micoli et al. 2021). GMMAs promise to be a vaccine design tool since they can induce strong immunogenicity, which can even be genetically manipulated to modulate systemic reactogenicity. These can induce strong immunogenicity due to the native presence of LPS, Lpps, and PGs (Hu et al. 2022; Mancini et al. 2021). Immunogenicity has been related to the size of the vesicles and their ability to present different antigens in a bacteria-like environment. However, the mechanisms of action are still being studied (Mancini et al. 2021). Interestingly, through genetic manipulation and loading strategies such as chemical conjugation, the risk of systemic reactivity of GMMA can be regulated, and functions for transporting polysaccharides or heterologous protein antigens can be conferred (Di Benedetto et al. 2021; Gerke et al. 2015; Mancini et al. 2021). GMMA production widely involves the growth of the hypervesiculating strain in a bioreactor and two sequential filtration processes (Gerke et al. 2015).
A variety of GMMA are in development, and some are in more advanced states, which have been tested in preclinical and clinical trials (Table 1) (Gerke et al. 2015; Mancini et al. 2021). GMMA have been tested as a platform for loading antigens such as hemagglutinin, associated with influenza A virus, or glycoproteins from rabies virus, enhancing humoral and antigen-specific cell-mediated responses in mice (Hu et al. 2022). Instead, the genetic modification of Shigella sonnei led to the production of GMMA with reduced endotoxicity LPS while maintaining the virulence plasmid associated with the O-antigen (Gerke et al. 2015). The GMMA from S. sonnei have been tested in phases I and II, and it is well tolerated after intramuscular, intranasal, and intradermal administration (Launay et al. 2017). Moreover, the vaccine based on GMMA derived from the engineered meningococcal B strain, which reduces CPS and LPS, has been tested in clinical trials, being safe and showing protection in volunteers (Keiser et al. 2010).
BEVs carrying therapeutic recombinant proteins have generated attention, especially if they can be directed to specific tissues. The fusion of proteins or molecules to the BEVs to be recognized by specific receptors in specific cells is a common strategy. For example, antigens can be directed to the periplasmic space to be packaged and released as BEVs (Kesty and Kuehn 2004; Muralinath et al. 2011). Streptococcus proteins have been fused with OmpA from the E. coli in the periplasm and successfully packaged into BEVs to induce the production of functional antibodies in immunized mice (Fantappiè et al. 2014). Furthermore, by employing bioconjugation systems or plug and display, the production of OMVs loaded with spike receptor binding domains (RBD) of SARS-CoV-2 and phosphotriesterases has been achieved (Alves et al. 2015; Jiang et al. 2022).
In recent studies, BEVs have been used to carry cancer-specific epitopes or non-coding RNAs (Grandi et al. 2018; Zhang et al. 2019a, b) to lasting antitumor immune response and might inhibit the growth of different tumoral cells (Chen et al. 2020a; Zhang et al. 2019a, b), being important in cancer immunotherapy (Zhang et al. 2019a, b). Similarly, E. coli BEVs loaded with chemotherapeutic drugs have been used to eradicate breast cancer cells in mice (Li et al. 2023). Moreover, Lacticaseibacillus paracasei and L. plantarum BEVs only loaded with endogenous cargo inhibited the proliferation of colorectal cancer and had antidepressant effects, respectively (Choi et al. 2019; Shi et al. 2022). These results open the doors to the development of new biotherapeutics.
BEVs have been tested as antimicrobial agents and biomarkers in other biotechnological areas. For example, L. plantarum MVs have been found to inhibit the growth of Shewanella putrefaciens in tuna (Lee et al. 2021), and Helicobacter pylori OMVs coated with nanoparticles of poly(lactic-co-glycolic acid), prevents the adhesion of H. pylori to epithelial cells (Zhang et al. 2019a, b). Tuberculosis could be diagnosed by detecting specific sRNAs, such as Asdes and MTB-miR5, encapsulated in M. tuberculosis MVs that could be obtained from blood samples of infected patients (Lu et al. 2021). Furthermore, BEVs can be used to support enzymes that degrade contaminating compounds (Alves et al. 2015; Thoma et al. 2018).
Around 802 clinical trials involving EVs (exosomes, ectosomes, microvesicles, or OMVs) have been registered at clinicaltrials.gov, of which only 48 are related to OMVs. Although using BEVs in various areas is promising, different limitations condition their application (Bitto and Kaparakis-Liaskos 2017). One of them is the heterogeneity in sizes and composition, which can bias the results in clinical trials (Alves et al. 2015; Bitto et al. 2017; García-Manrique et al. 2018). Another limitation is the presence of immunogens that can cause adverse effects, mainly when the purpose of BEVs is different from modulating immune responses (Balhuizen et al. 2021a; Bitto and Kaparakis-Liaskos 2017). Among the main concerns, the low production of BEVs become a critical factor for the development of therapeutics due to the high concentrations required to achieve efficiency in treatments and cover the demands (Balhuizen et al. 2021a; Hu et al. 2020; Jahromi and Fuhrmann 2021; Morishita et al. 2021; Reimer et al. 2021; van de Waterbeemd et al. 2013a). Further, more research is still needed on the use of vesicles as therapeutic agents, their toxicology, as well as on pharmacodynamics and pharmacokinetics of BEVs (García-Manrique et al. 2018; Jahromi and Fuhrmann 2021).
Strategies to increase the productivity of BEVs
The productivity of EVs is typically low compared with the biomass, representing less than 1%. Different strategies to increase the productivity of BEVs have been explored over the last fifty years (Fig. 4) (Aytar-Çelik et al. 2022; Balhuizen et al. 2021a), such as genetic modifications of the cell envelope and culture conditions in stressful environments (Fig. 4B, C, Supplementary 1A, B, C). An strategy used since the 1970s, is the production and recovery of artificial BEVs, highlighting the sonication (Balhuizen et al. 2021a) (Fig. 4D).
Strategies to increase the productivity of BEVs and updated bibliometric data on these strategies. (A) Bibliometrics data associated with abundant BEV production strategies over the last fifty years. (B) Publications report related to the genetic modifications of relevant proteins related to abundant vesiculation. (C) Publications report related to environmental and chemical stress conditions that lead to the abundant release of BEVs during bacteria culture. (D) Publications report related to methodologies employed by the artificial BEVs production
Molecular strategies to increase BEVs formation: deletion, mutation, knock-out, or gene overexpression
Different genetic modifications have been evaluated as a strategy to increase BEV production, mostly based on the mutation, deletion, or knock-out of genes associated with proteins or processes of the cell envelope (Fig. 4B; Table 2) (Balhuizen et al. 2021a). One of the most used protein targets for developing strains with hypervesiculation is the Tol-Pal system (Pérez-Cruz et al. 2016; Reimer et al. 2021; Song et al. 2021; Takaki et al. 2020). This system contains a set of five distinct proteins that connect the IM with the PG and the OM, maintaining the structure of the cell envelope and participating in the cell division of Gram-negative bacteria (Mozaheb and Mingeot-Leclercq 2020; Zingl et al. 2020). TolQ, TolR and TolA form a complex in the IM and interact with TolB through TolA. TolB in the periplasm, binds to the Pal lipoprotein, which interacts with the PG via non-covalent bonds (Fig. S2) (Mozaheb and Mingeot-Leclercq 2020; Zingl et al. 2020). Defects in the components of this system lead to the formation of BEVs due to the dissociation of PG and CMs (Takaki et al. 2020).
The deletion of the tolB gene in the B. agrestis JCM 1090 T increased the release of vesicles 17-fold compared to the wild strain, promoting BEV formation from the poles of the cell, at the sites of cell division and on the lateral surfaces (Takaki et al. 2020). Similarly, the tolA modification in E. coli K-12 BW25113 and E. coli BL21(DE3) increased the release of BEVs four and 13-fold, respectively, relative to wild-type strains (Reimer et al. 2021; Song et al. 2021). The tolR mutation of E. coli Nissle 1917 increased BEV production to 52-fold compared to the wild strain (Pérez-Cruz et al. 2016), also causing differences in the protein composition, and heterogeneity in size and shape of BEVs (Pérez-Cruz et al. 2016). The physical heterogeneity was derived in a loss in the interaction capacity of the BEVs of E. coli Nissle 1917 with epithelial cells (Pérez-Cruz et al. 2016). Interestingly, the variability in size and morphology in BEVs also has been observed in E. coli K-12 BW25113 ΔtolA and B. agrestis ΔtolB strains, obtaining O-IMVs, multilamellar vesicles (M-OMVs), multivesicular (G-OMVs), and partially circularized vesicles (Pérez-Cruz et al. 2016; Reimer et al. 2021; Takaki et al. 2020).
Another group of key genes for generating bacteria with hypervesiculation are those associated with the synthesis of Lpps (Schwechheimer et al. 2014, 2015; Schwechheimer and Kuehn 2013). The Lpps, also known as cross-linking proteins, correspond to peripheral membrane proteins with a hydrophobic tail that serves as an anchor to the lipid bilayer (Mathelié-Guinlet et al. 2020; Mozaheb and Mingeot-Leclercq 2020). In Gram-positive bacteria, Lpps are further attached to the cytoplasm via an N-terminal lipid residue, and their functions include maintaining the integrity and organization of the CM (Wang et al. 2020; Wang et al. 2021a, b). The mutation of the lgt gene coding for the lipoprotein diacylglycerol transferase increased the fluidity of the plasmatic membrane, doubling or tripling MVs production by the S. aureus JE2 Δlgt and S. aureus Newman Δlgt strains with respect to wild-type strains (Wang et al. 2020). The BEVs released from mutated bacteria presented smaller sizes and alterations in protein content, such as a reduction of Pore-forming toxins (PFTs) used by S. aureus as virulence factors (Wang et al. 2020). In Gram-negative bacteria, Lpps are anchored to PG via a C-terminal lysine (Zingl et al. 2020). Mutation of the nlpI gene encoding the OM Lpp NlpI in E. coli Nissle 1917 and E. coli K-12 BW25113 increased vesiculation two-fold and six-fold, respectively, compared to wild-type strains (Ojima et al. 2018; Thomas et al. 2022). NlpI participates in cell division and in the negative regulation of PG endopeptidases, and its mutation reduces the crosslinking of Lpp and PG by up to 40%, increasing indirectly the formation of BEVs (Ojima et al. 2018; Schwechheimer et al. 2015).
On the other hand, deletion of the degP increased the production of OMVs up to eight-fold without affecting growth of E. coli. The degP codes for a periplasmic chaperone-protease that removes misfolded proteins in the cell envelope (Ojima et al. 2018; Schwechheimer et al. 2015). The mutation of degP leads to the accumulation of proteins in the cell wall, generating cellular stress and causing the production of BEVs to release accumulated proteins (Ojima et al. 2018; Schwechheimer and Kuehn 2013).
Interestingly, in BEVs from E. coli ΔnlpI and E. coli ΔdegP the loading with recombinant protein OmpW-GFP (GFP, green fluorescent protein) is differential, being higher in vesicles from E. coli ΔnlpI (Ojima et al. 2018). This suggests the importance of hypervesiculation and the incorporation of cargo molecules.
In wild strains of Gram-negative bacteria, the vacJ (mlaA in E. coli), in conjunction with ybr or mla (B, C, D, E and F), encode for subunits of the ABC transporter VacJ/Yrb, whose function is to maintain the OM asymmetry (Malinverni and Silhavy 2009; Roier et al. 2016). Studies on the biogenesis of OMVs in Haemophilus influenzae and V. cholerae revealed that the deletion or reduction in the expression of the vacJ and yrb cause the accumulation of phospholipids in the outer leaflet of the OM. To maintain membrane asymmetry, i.e., LPS in the outer leaflet and phospholipids in the inner leaflet, bacteria secrete phospholipids through OMVs (Roier et al. 2016).
Double mutants of E. coli K-12 BW25113 (ΔmlaE:ΔnlpI) showed an increase of BEVs by 30-fold when compared with the wild-type strain, where the release of vesicles was associated with the reduction of cross-links between PG and Lpp, as well as with the accumulation of phospholipids in the cell OM (Ojima et al. 2020, 2021). In addition, the mutant E. coli ΔmlaE:ΔnlpI presented elongated cells, and BEVs generated mainly from the poles of the cells with sizes larger compared to the wild-type strain (Ojima et al. 2021). While deleting the lysis module (Dlm) in E. coli ULS153 increased up to fourfold the production of OMVs (Pasqua et al. 2021). Dlm consists of an operon of four genes (essD, ybcS and rzpD/rzoD) encoding a holin (S), an endolysin (R), and two spanins (Rz/Rz1), which contribute to the release of PG residues (Pasqua et al. 2021). The holin generates small holes in the internal CM for the passage to the periplasm of endolysin and spanins, which are responsible for causing cell lysis (Pasqua et al. 2021; Turnbull et al. 2016).
In addition, another mechanism for the abundant production of BEVs is the overexpression of the OmpT protease (Premjani et al. 2014). In Enterohemorrhagic E. coli (EHEC), this modification released 40-fold more OMVs than the wild-type strain (Premjani et al. 2014). It is considered that high levels of OmpT in the OM altered the number of contacts between the PG and the OM via proteolysis, leading to membrane detachment and the formation of BEVs (Premjani et al. 2014). The overproduction of the OmpT protease impacts the characteristics of the OMVs, which have smaller diameters, and lower protein and lipid content compared to the OMVs of the wild-type strain (Premjani et al. 2014).
In general, from the productive point of view, the modifications in the tolB and tolR genes of the Tol-Pal system, and the double knock-out of the nlpI and mlaE genes, are the most promising systems to obtain strains with hypervesiculation (Fig. 4B; Table 2). However, although genetic modification makes it possible to obtain strains that overproduce BEVs, these are heterogeneous in size, composition, and morphology, so it is necessary to evaluate the advantages and disadvantages in the development of new biotherapeutics, vaccines, and delivery systems.
Culture strategies to obtain abundant BEVs
Throughout evolution, bacteria have acquired various adaptation mechanisms (Guan et al. 2017; Hews et al. 2019), including the ability to sense environmental changes and use them as inducers of various cellular responses to stress (Hews et al. 2019). The cell envelope is the first line of defense of bacteria, so there are specific envelope stress response systems (ESRS) that ensure cellular homeostasis, maintain its integrity and fluidity (Eberlein et al. 2018; Guan et al. 2017), and sense extracellular stress and perturbations in the periplasm (Hews et al. 2019; Laloux and Collet 2017).
The release of BEVs has been cataloged as a stress response system in the envelope (Klimentová and Stulík 2015; McBroom and Kuehn 2007; Mozaheb and Mingeot-Leclercq 2020), which maintains the composition of the periplasm in balance through selective packaging and secretion of harmful material (McBroom and Kuehn 2007). This ESRS may act as an immediate response mechanism protecting the OM (Eberlein et al. 2018; Manning and Kuehn 2011). Vesiculation involves the extraction of misfolded proteins from the cell, changes in the composition of the OM, or neutralize the deleterious effect of toxic compounds, allowing the bacteria activate ESRS with complex signaling pathways (Eberlein et al. 2018; Manning and Kuehn 2011; Mozaheb and Mingeot-Leclercq 2020). Bacteria also use the release of BEVs to export regulatory proteins and proteolytic products accumulated in the envelope derived from other ESRS (McBroom and Kuehn 2007).
Since, bacteria respond to external stimuli by modulating the composition of the cell envelope and producing BEVs (Klimentova et al. 2019; Klimentová and Stulík 2015; Yokoyama et al. 2021), stressful conditions has been used to increase vesiculation (Klimentová and Stulík 2015; Wang et al. 2021a, b). Stress factors, evaluated as strategies to increase the release of BEVs, are divided into two categories, environmental stress, and chemical stress (Fig. 4C and Table 3).
Cultivation at acidic pH
The cultivation of S. mutans, Salmonella enterica, and Francisella tularensis at pH between 5.3—5.8, increases the number of BEVs up to tenfold higher than cultures at neutral pH (Table 3) (Bonnington and Kuehn 2016; Cao et al. 2020; Klimentova et al. 2019).
The size and morphology of BEVs produced in acidic environments vary according to the bacteria; for example, the S. mutans MVs were smaller at pH 5.5 than 7.5 (Cao et al. 2020). The modification of the pH and reduction in the concentration of Mg2+ (pH 5.8 and 10 μM of Mg2+), leads to the release of BEVs 20 nm larger by S. enterica, compared to the control (pH 7.6 and 10 mM Mg2+) (Bonnington and Kuehn 2016). Meanwhile, F. tularensis releases OMVs in the form of nanotubes at pH 5.3 (Klimentova et al. 2019), which are used by bacteria as connection bridges with other cells (Gill et al. 2019; Klimentova et al. 2019).
The MVs obtained from S. mutans at acidic pH presented fewer proteins, and ABC transporters were identified as the most important transport pathways during the bacteria growth under stress conditions (Cao et al. 2020). The reduction in the number of proteins in the MVs of S. mutans was attributed to these transporters (Cao et al. 2020). Likewise, the OMVs of F. tularensis show a reduction in the concentration of proteins associated with the biosynthesis of O-antigen, lipid A, phospholipids, and fatty acids, reflecting changes in the protein and lipid composition of the OM in response to environmental variation (Klimentova et al. 2019).
Cultivation at low and high temperatures
Thermal stress is another factor that increases BEV production (Klimentová and Stulík 2015; Mozaheb and Mingeot-Leclercq 2020). When the temperature varies, different cellular components suffer alterations, like the accumulation of misfolded proteins and modifications in the acyl chains of phospholipids, which are among the most common (Eberlein et al. 2018; Klimentová and Stulík 2015; Schwechheimer et al. 2013).
Vesiculation modulated by thermal stress has been evaluated in Bordetella pertussis, Bordetella bronchiseptica, F. tularensis, P. putida, P. aeruginosa, A. baylyi and S. aureus, with increases in the number of vesicles up to 39-fold compared to control conditions (Table 3) (Baumgarten et al. 2012; De Jonge et al. 2021; Fulsundar et al. 2014; Klimentova et al. 2019; MacDonald and Kuehn 2013; Wang et al. 2021a, b). Although hypervesiculation is frequent in cultures with temperature increases, it has been observed that strains such as S. aureus release a greater number of vesicles below 30 °C (Wang et al. 2021a, b). BEVs derived from the cultivation of bacteria under thermal stress present spherical morphologies (Balhuizen et al. 2021b; Baumgarten et al. 2012; De Jonge et al. 2021). However, F. tularensis, for example, secretes long tubular BEVs when grown at 42 °C, and spherical BEVs of heterogeneous sizes and nanotubes when grown at 25 °C (Klimentova et al. 2019).
BEV composition changes according to the bacterium and heat shock condition (Balhuizen et al. 2021b; De Jonge et al. 2021; Klimentova et al. 2019; Wang et al. 2021a, b). The inactivation by heat shock of B. pertussis and B. bronchiseptica resulted in OMVs with protein patterns like the controls, but a higher amount of protein, an increase in phosphatidylglycerol and lysophospholipids, and a reduction in phosphatidylethanolamine was found. The increase in lysophospholipids, characterized by having a single fatty acid, could also lead to a decrease in stability in the BEVs of B. pertussis and B. bronchiseptica (Balhuizen et al. 2021b). On the other hand, the culture of F. tularensis at 25 °C and 42 °C generated BEVs with protein compositions different from each other and from the control (Klimentova et al. 2019). For S. aureus the reduction in temperature favored the incorporation of LTA in the MVs (Wang et al. 2021a, b).
Cultivation under oxidative stress
Oxidative stress is a condition associated with the accumulation of reactive oxygen and reactive nitrogen species in cells. It causes damage to DNA, membrane lipids, and proteins and results in cell death (Ezraty et al. 2017). As defense systems, bacteria synthesize neutralizing enzymes of reactive species, activate stress responses, and release BEVs (Ezraty et al. 2017; Mozaheb and Mingeot-Leclercq 2020).
The contact of S. aureus, F. tularensis, and P. aeruginosa with sublethal concentrations of hydrogen peroxide (H2O2) or ciprofloxacin, the increase in dissolved oxygen tension in cultures of N. meningiditis and Campylobacter jejuni, and the growth of P. aeruginosa in denitrifying and anoxic conditions, triggered the abundant production of BEVs, up to six-fold higher than controls (Table 3) (Gerritzen et al. 2018; Godlewska et al. 2019; Klimentova et al. 2019; Toyofuku et al. 2014; Wang et al. 2021a, b). The abundant vesiculation could be associated with the accumulation of misfolded proteins in the periplasm due to the presence of H2O2 (MacDonald and Kuehn 2013), or to the synthesis of pyocins in anoxic conditions, which might induce disruptions in the IM, PG, and OM links (Toyofuku et al. 2014). The morphology of BEVs produced under oxidative stress depends on the parental bacterium, having OMVs in the form of nanotubes from F. tularensis (Klimentova et al. 2019), and spherical vesicles with diameters between 20 and 110 nm, from P. aeruginosa, S. aureus, N. meningitidis, and C. jejuni (Gerritzen et al. 2018; Godlewska et al. 2019; Toyofuku et al. 2014; Wang et al. 2021a, b).
BEV composition changes according to oxidative stress type and bacteria. The H2O2 changes the composition of the LPS in OMVs of P. aeruginosa (MacDonald and Kuehn 2013). While ciprofloxacin reduces the amount of LTA in MVs in S. aureus (Wang et al. 2021a, b). C. jenuni culture with a high TOD released OMVs loaded with PorA, OMP that alters the permeability of the CM, and Cjj81176_0211, an iron-binding protein (Godlewska et al. 2019). Whereas P. aeruginosa, under anoxic conditions, released vesicles enriched with the chaperone GroEL and proteins for pyocin assembly (Toyofuku et al. 2014).
Cultivation under osmotic stress
Osmotic stress is another condition related to high vesiculation, caused by changes in pH and the addition of salts to equilibrate it (Baumgarten et al. 2012; Lee et al. 2018). Exposure of L. monocytogenes to NaCl, 0.5 M, increases the release of vesicles, obtaining spherical nanostructures without appreciable differences in shape and size compared to the control (Lee et al. 2018). Significant increases of OMVs were also reported in P. putida cultures upon 2 M of NaCl (Baumgarten et al. 2012).
The increase in the concentration of NaCl in the extracellular environment of L. monocytogenes activated the responses to stress σB and SOS, being found in the MVs proteins related to both stress systems, such as osmolyte transporters or RecA (Lee et al. 2018). Likewise, the stress condition increased the concentration of virulence factors and proteins related to osmotic stress, such as GbuA (Lee et al. 2018). Meanwhile, vesicles from P. putida showed a significant increase in the degree of saturation of fatty acids with respect to the parental cell, coupled with an increase in the hydrophobicity of the cell surface and in the capacity of the bacteria to form biofilms (Baumgarten et al. 2012). Therefore, the increase in vesiculation in L. monocytogenes and P. putida could be related to alterations in membrane fluidity, protein folding, and DNA changes triggered by the presence of salts (Mozaheb and Mingeot-Leclercq 2020). Based on the protein composition of the MVs of L. monocytogenes, the secretion of vesicles might be a survival mechanism through which the bacterium adapts to natural environments such as the small intestine and duodenum, in which the osmotic pressure varies (Lee et al. 2018). Whereas 1% and 2% of NaCl in cultures of S. aureus reduce MV secretion compared to the control (culture without NaCl) due to a thickening of the cell wall that prevents the exit of the MVs (Wang et al. 2021a, b).
Cultivation under nutrient depletion
Nutrient depletion has been evaluated as an inducer of vesiculation in N. meningitidis for the formulation of vaccines against Meningococcus Group B (Gerritzen et al. 2019a; van de Waterbeemd et al. 2013b). For instance, the cysteine limitation increases vesiculation 12-fold compared with the control (van de Waterbeemd et al. 2013b). Likewise, the culture of N. meningitidis under reduced concentrations of sulfate increases the number of vesicles two-fold in relation to a condition of cysteine depletion (Gerritzen et al. 2019a).
The morphology of the vesicles secreted by N. meningitidis due to nutrient depletion does not present significant variations with respect to the controls (Gerritzen et al. 2019a; van de Waterbeemd et al. 2013b). Additionally, the OMVs generated from the depletion of cysteine have a similar composition to OMVs released by the bacteria during infection, making it possible to use them in the formulation of vaccines against Meningococcus Group B (van de Waterbeemd et al. 2013b). However, the composition of OMVs derived from sulfate depletion presents an enrichment of phospholipids and low concentrations of LPS, which affects their application in vaccine production due to the reduction of PAMPs (Gerritzen et al. 2019a). Transcriptomic analysis of N. meningitidis cultured under cysteine-limiting conditions suggests that amino acid depletion alters iron-sulfur protein biogenesis, leading to increased intracellular iron and oxidative stress (van de Waterbeemd et al. 2013b). While sulfate depletion triggers an increase in phospholipid biosynthesis, the accumulation of which may be associated with BEV biogenesis (Gerritzen et al. 2019a). For S. aureus, cultured in the presence of the iron chelator 2,2-dipyridyl leads to the production of MVs three-fold higher than the control condition (Wang et al. 2021a, b). The release of BEVs is probably related to acquiring the nutrient (Wang et al. 2021a, b). Table 3 presents stress conditions that increase the production of BEVs, which might be effective for the bioprocesses design.
Cultivation with chemical compounds
The contact of bacterial cells with chemical compounds, such as solvents, detergents, or chelators, alters the composition and integrity of the membranes (Mozaheb and Mingeot-Leclercq 2020). As a stress-defense mechanism, bacteria responses such as cis/trans isomerization of fatty acyl residue in membrane phospholipids, ESRS activation, and vesicle release (Eberlein et al. 2018; Mozaheb and Mingeot-Leclercq 2020). Compounds such as Ethylenediaminetetraacetic acid (EDTA), which sequester calcium and magnesium ions, destabilize the OM, altering its interaction with PG and IM, and triggering the release of BEVs (Balhuizen et al. 2021a; Baumgarten et al. 2012; van de Waterbeemd et al. 2012). EDTA-induced vesicles tend to be less stable than those released spontaneously (Balhuizen et al. 2021a). Ionic and non-ionic detergents such as SDS (Sodium Dodecyl Sulfate) and deoxycholic acid, respectively, provoke increased vesiculation and detoxified OMVs by removing LPS, but with the disadvantage of vesicle aggregation (Gnopo et al. 2017; van de Waterbeemd et al. 2010, 2012). Currently, the generation of genetically modified strains with LPS of attenuated toxicity seeks to replace the production of BEVs mediated by detergents (Gerritzen et al. 2019b; van de Waterbeemd et al. 2013a).
The 1-octanol, ethanol, D-cycloserine, lysine, and sucrose fatty acid esters also increase the production of BEVs, triggering alterations in the PG (Table 3) (Abe et al. 2021; Baumgarten et al. 2012; Wang et al. 2021a, b; Yokoyama et al. 2021). D-cycloserine, analogous to D-alanine, acts as an inhibitor of PG synthesis, altering the integrity of the envelope of P. aeruginosa (MacDonald and Kuehn 2013). Lysine reduces the expression of HM1357, which is responsible for controlling the transcription of genes related to PG synthesis in Shewanella vesiculosa (Yokoyama et al. 2021). The sucrose fatty acid esters, a surfactant with antimicrobial activity, activate autolysins already expressed in the cell wall of B. subtilis, triggering PG degradation and cell death (Abe et al. 2021). The sucrose fatty acid esters cause the greatest increases in vesiculation (Abe et al. 2021). The composition of BEVs seems to be closely linked to the stress response mechanisms activated by the cell in the presence of the above mentioned compounds, where lysine sensor proteins (HM1275) and autolysin were found as part of their response protein profile (Abe et al. 2021; Yokoyama et al. 2021). For instance, the presence of BEVs maintains the viability of B. subtilis around 90% in presence of sucrose fatty acid esters up to 40 μg/mL, and the load of HM1275 in BEVs of S. vesiculosa was related to an increase in the ability of the bacteria to form biofilms (Abe et al. 2021; Yokoyama et al. 2021). While, with the use of EDTA, an increase in the degree of lipid saturation was reported (Baumgarten et al. 2012).
Cultivation with antibiotics
Antibiotics such as gentamicin, ciprofloxacin, chloramphenicol, polymyxin B, and ampicillin, as well as antimicrobial peptides such as cathelicidins or Host Defense Peptides (HDPs) are vesiculation inducers (Balhuizen et al. 2021b; Bos et al. 2021; Fulsundar et al. 2014; Godlewska et al. 2019; Kim et al. 2020b). Antibiotic-mediated vesicle generation is related to the mechanism of action of the compounds on cells. Ciprofloxacin is an inhibitor of DNA replication that causes activation of the SOS response related to the explosive cell lysis and is correlated with the increase of BEVs in E. coli (Bos et al. 2021; Turnbull et al. 2016). Ampicillin, an antibiotic of the β-lactam family applied to S. aureus cultures, leads to the most significant increases in vesiculation, 22-fold compared to the control (Kim et al. 2020b). The polymyxin B used in C. jejuni (Godlewska et al. 2019) and HDPs in B. bronchiseptica cultures also moderately increase vesicle production (Balhuizen et al. 2021b). While gentamicin, a polycationic antibiotic that, in addition to inhibiting protein synthesis, interacts with OM, causing alterations in LPS due to the displacement of Ca2+ and Mg2+, being responsible for the increase in vesiculation sites of A. baylyi (Fulsundar et al. 2014; Mozaheb and Mingeot-Leclercq 2020). Morphology and properties of the BEVs of B. bronchiseptica in the presence of cathelicidin derived in spherical and tubular vesicles with average sizes between 20 and 40 nm reduce its stability compared to the spontaneous OMVs (Balhuizen et al. 2021b).
The composition of BEVs induced by antibiotics, in most cases, presents an increase in protein concentration, such as enzymes are related to the degradation of the applied antibiotic (Fulsundar et al. 2014; Godlewska et al. 2019; Kim et al. 2020b). The addition of ampicillin to E. coli cultures significantly increases the amount of Pal lipoprotein, which, together with LPS, increases the toxicity of BEVs (Michel et al. 2020). LPS in BEVs from A. baylyi increases in response to gentamicin, as well as DNA concentration (Fulsundar et al. 2014). The increased accumulation of other lipids, such as phosphatidylglycerol in BEVs from B. bronchiseptica, also has been detected (Balhuizen et al. 2021b), changing its interaction with macrophages, reducing the expression of cytokines, and a loss of virulence (Balhuizen et al. 2021b).
Considerations for the production of BEVs
One environmental aspect important in the production of BEVs is the culture medium, which can be key in the abundant vesiculation that might determine their composition (McCaig et al. 2016; Yokoyama et al. 2021). The culture of Haemophilus paresuis in Brain Heart Infusion increases vesiculation compared to Casman’s broth base and Soy Trypticase media (McCaig et al. 2016). Likewise, the culture of S. vesiculosa in Lysogenic Broth (LB) increases the release of BEVs 39-fold compared to Bacto Marine Broth and eight-fold compared to M79 (modified DSMZ medium). The culture of H. paresuis in LB was also accompanied by an increase in the production of protein P49, which is abundant in BEVs (Yokoyama et al. 2021). Although the function of this protein is not known, its presence in the vesicles was associated with the non-canonical protein secretion system T2SS and particularly with the GspD2 protein, a possible OM channel responsible for the loading of molecules in the BEVs. The P49 protein is also a candidate to be fused with proteins to be transported and loaded in S. vesiculosa vesicles (Chen et al. 2020b). Unlike B. pertussis and B. bronchiseptica cultured in Verwey, Stainer-Scholte, and Thalen-IJssel media, influence the protein composition of OMVs, finding the receptor FauA (siderophore) in vesicles produced in Verwey, and the Zn receptor, ZnuD, in vesicles derived from Stainer-Scholte, and Thalen-IJssel media (De Jonge et al. 2021), attributed to the low availability of iron in those media (De Jonge et al. 2021).
The mode of operation of the bioprocess and the incubation time also increases the production of BEVs from some bacteria (Gerritzen et al. 2019b; Richter et al. 2021b). The production of vesicles by N. meningiditis in stirred tank reactors operated in continuous mode increases the production of BEVs by nine-fold compared to batch culture (Gerritzen et al. 2019b). While the E. coli BL21 DE3 culture for seven days leads to a higher concentration of BEVs compared to the culture for two days (Richter et al. 2021b). In the latter case, it is assumed that extending the culture time leads the bacteria to a starvation state resulting in a high release of BEVs (Richter et al. 2021b).
Strategies for the formation and recovery of artificial BEVs
Although various methods for abundant vesicle formation during bacterial culture have been tried, large-scale production of BEVs remains challenging. Various strategies have been implemented to generate artificial vesicles to solve this drawback. These strategies involve the use of chemical or physical processes that trigger the death or lysis of bacteria, releasing essential components, like lipid bilayers, proteins, and nucleic acids, for the reconstitution of BEVs (Fig. 4C and 4G) (Hahm et al. 2021; Park et al. 2021).
Artificials BEVs production by sonication
Sonication is the most used method in the production of artificial vesicles (Fig. 4D). (Hozbor et al. 1999; Park et al. 2021; Mougenot et al. 2022). Once the cells are isolated, they are resuspended in a buffer (pH between 8 and 8.5) and pretreated with EDTA and lysozyme (Hozbor et al. 1999; Park et al. 2021), weakens the cell envelope and degrading PG, respectively, generating spheroplasts (Li et al. 2021; Park et al. 2021). Protocols include the IM remotion by detergents such as Sarkosyl and cytosolic components by increasing the pH. Then, OMs are purified and sonicated at mild intensities (Fig. S3A) (Park et al. 2021). Sonication increased the number of BEVs compared to spontaneous release about 40-fold in E. coli (Park et al. 2021). In general, sonication produces spherical BEVs of around 50—150 nm and 150 and 250 nm (Hozbor et al. 1999; Park et al. 2021), with decreasing compounds such as LPS (Park et al. 2021). The protein profiles differ with respect to spontaneous OMVs, highlighting a higher accumulation of OMPs, the reduction of cytosolic proteins, and a lower capacity to protect cargo molecules (McCaig et al. 2016).
Artificial BEV production by extrusion
Extrusion is another methodology to produce artificial BEVs, using strategies such as the generation of protoplasts (Fig. S3B) (Harisa et al. 2020; Kim et al. 2015b). Extrusion involves the passage of protoplasts through a series of polycarbonate membranes of different sizes (10, 5, and 1 µm), obtaining Protoplast-derived nanovesicles (PDNVs) (Kim et al. 2015b). Then, the PDNVs are purified by density gradient ultracentrifugation (Kim et al. 2015b). The PDNVs have represented 200-fold higher than the spontaneous BEVs in E. coli. PDNVs present spherical morphologies with average diameters of 114 ± 10 nm, harboring cytoplasmic proteins and lacking OM components, such as LPS or OmpA (Kim et al. 2015b).
The production of vesicles from ghost bacteria has an opposite approach to that of protoplasm (Fig. S3B). In this strategy, the first step consists of generating cell envelopes devoid of cytoplasmic content using the lysis by the E gene (bacteriophage PhiX174) and/or the sponge-like protocol (Harisa et al. 2020; Youssof et al. 2019). The lysis method involves the genetic modification of the bacteria with the E gene, encoding a membrane protein that oligomerizes in a transmembrane tunnel allowing the cytoplasmic content release, and leaving the cell envelope intact (Harisa et al. 2020; Langemann et al. 2010). The sponge-like protocol method uses agents such as sodium hydroxide, calcium carbonate, SDS, H2O2, and ethanol, which degrade genetic material and other cellular components and promote their release through an altered cell envelope (Youssof et al. 2019). Once the ghost cells are obtained, they are extruded through membranes with pore sizes of 100 nm, obtaining vesicles called bacteriosomes (Harisa et al. 2020).
Artificials BEVs production by high pressures
The production of BEVs at high pressures (Fig. S3C), is a method analogous to the extrusion process. In the pressure method, self-assembling vesicles, or bacterial biomimetic vesicles (BBVs) are formed after passing the membranes through a homogenizer with small holes that deform the cell envelope in the form of buds (Hua et al. 2021; Li et al. 2021). Through this method, the number of vesicles produced by K. pneumoniae and E. coli has increased to 88 and 98-fold, respectively, compared with spontaneously released OMVs (Hua et al. 2021; Li et al. 2021). The BBVs of E. coli were also loaded with the fusion protein ClyA-IL10, increasing the amount of ClyA-IL10, 31-fold higher compared to IL10-OMVs (Hua et al. 2021). In addition to increasing vesicle production, this method improves the incorporation of cargo molecules.
The morphology of the BBVs, obtained at a pressure of 1200 bar, is mainly rounded with average sizes between 180 and 210 nm, and stable for at least five weeks at room temperature, 4 °C, and − 20 °C (Hua et al. 2021; Li et al. 2021). At lower pressures (200 to 800 bar), the vesicles present incomplete structures, ruling out these operating conditions (Hua et al. 2021). The protein composition of BBVs shows a significant increase in IM, OM, and periplasmic proteins and a reduction in cytoplasmic components, including nucleic acids (Hua et al. 2021; Li et al. 2021). Both K. pneumonia and E. coli BBVs, conceived for immunogenic treatments (production of vaccines and anticancer agents, respectively), managed to activate the immune response of the host organisms where they were evaluated, providing a new alternative to produce vaccines and immunotherapy for cancer (Hua et al. 2021; Li et al. 2021).
Quantification of BEVs
Quantification is crucial for evaluating the efficiency of BEV production strategies (Klimentová and Stulík 2015). Various analytical methods can be used to quantify BEVs (Tables 2 and 3), which can be divided into direct and indirect (Bitto et al. 2021; Klimentová and Stulík 2015). Indirect methods, such as the determination of total protein and lipids, are the most common (Tables 2 and 3). These have the advantages of easy implementation, not requiring sophisticated equipment, and generally being a routine laboratory analysis. The most used protein assays are Bradford and bicinchoninic acid (BCA) (Bitto et al. 2021; Klimentová and Stulík 2015). Meanwhile, for the quantification of BEVs through lipids, the FM4-64 assay is widely reported (Klimentová and Stulík 2015). However, it is not always possible to correlate the protein and lipid content with the BEV quantity since the composition and size of the vesicles may vary depending on their production conditions, bacterial strain, and isolation method, among others (Hirayama and Nakao 2020; Bitto et al. 2021; Wei et al. 2022). Dry weight has been reported as a relative measure of vesiculation (Deatherage et al. 2009; Klimentová and Stulík 2015; McMahon et al. 2012). However, this technique can measure cellular material that does not correspond to vesicles (McMahon et al. 2012).
Direct methods, such as nanoparticle tracking Analysis (NTA), flow cytometry, transmission electron microscopy (TEM), or tunable resistive pulse sensing, are also used for vesicle quantification (Bitto et al. 2021; Goreham et al. 2019), among others. These methods are interesting because they allow the direct counting of particles, ensuring that changes in BEV composition would not affect possible comparisons between production processes (Bitto et al. 2021). However, the disadvantages lie in the need for expensive equipment and specialized staff (Goreham et al. 2019). It is necessary to continue the search for accessible methods that allow direct quantification of BEVs, limiting the biases introduced by indirect methods.
Concluding remarks
The biological relevance of BEVs has been recognized, moreover its use and biotechnological application continue to be in development. Here, we focus on strategies that increase vesicle production, inspired by BEV biogenesis mechanisms and functions, with significant increases in vesiculation. Having to mention that in several studies, different families of vesicles are observed; each of them might have different protein patterns and number of molecules inside, the lipid composition changes, and the nature of the vesicles varies according to the culture time, production time, and in response to the changes in environmental conditions suffered during production. Furthermore, we described different insults that cause hypervesiculating phenotypes. However, until now, mutants that do not vesiculate have been described, pointing out that this mechanism is constant in cells and could be manipulated and orchestrated as a cellular response.
A constant debate, with a view to the biotechnological use, is the low amounts of OMVs that are obtained from cultured bacteria at least two or three orders of magnitude with respect to the biomass. This is combined with the variation in the formation of vesicles and technical difficulties in the biophysical determination of their properties. Even more, during their purification, vesicles might aggregate or coalesce and are influenced by known purification methods. Some results published works may contain artifacts, or the physicochemical analysis only describes a part of a variety of BEVs that could be obtained in production processes.
Biotechnologically, it is still important to know the biological background of production to design OMVs with known and designed characteristics. In the future, the knowledge that might be generated about BEV production, the importance of environmental conditions on it, the kinetic analysis, as well as the use of in-depth lipidomic, proteomic, and microscopy techniques will allow improvement in the knowledge and description of the BEVs from relevant strains to clinical evaluations. Importantly, the sum of knowledge on their properties, and the application needs, could lead to controlled bioprocesses producing BEVs with known morphologies, up to the design of composition, shape, and size that will ensure their quality, safe, and effectiveness.
Data availability
No datasets were generated or analyzed during the current study.
References
Abe K, Toyofuku M, Nomura N, Obana N (2021) Autolysis-mediated membrane vesicle formation in Bacillus subtilis. Environ Microbiol 23(5):2632–2647. https://doi.org/10.1111/1462-2920.15502
Aktar S, Okamoto Y, Ueno S, Tahara YO, Imaizumi M, Shintani M, Miyata M, Futamata H, Nojiri H, Tashiro Y (2021) Incorporation of plasmid DNA into bacterial membrane vesicles by peptidoglycan defects in Escherichia coli. Front Microbiol 12:747606. https://doi.org/10.3389/fmicb.2021.747606
Alfini R, Brunelli B, Bartolini E, Carducci M, Luzzi E, Ferlicca F, Buccato S, Galli B, Lo Surdo P, Scarselli M, Romagnoli G, Cartocci E, Maione D, Savino S, Necchi F, Delany I, Micoli F (2022) Investigating the role of antigen orientation on the immune response elicited by Neisseria meningitidis factor H binding protein on GMMA. Vaccines. https://doi.org/10.3390/vaccines10081182
Alvarez CS, Giménez R, Cañas MA, Vera R, Díaz-Garrido N, Badia J, Baldomà L (2019) Extracellular vesicles and soluble factors secreted by Escherichia coli Nissle 1917 and ECOR63 protect against enteropathogenic E. coli-induced intestinal epithelial barrier dysfunction. BMC Microbiol 19(1):1–12. https://doi.org/10.1186/s12866-019-1534-3
Alves N, Turner K, Medintz I, Walper S (2015) Emerging therapeutic delivery capabilities and challenges utilizing enzyme/protein packaged bacterial vesicles. Ther Deliv 6(7):873–887. https://doi.org/10.4155/tde.15.40
Alves N, Moore M, Johnson B, Dean S, Turner K, Medintz I, Walper S (2018) Environmental decontamination of a chemical warfare simulant utilizing a membrane vesicle-encapsulated phosphotriesterase. ACS Appl Mater Interfaces 10(18):15712–15719. https://doi.org/10.1021/ACSAMI.8B02717
Arigita C, Jiskoot W, Westdijk J, van Ingen C, Hennink W, Crommelin D, Kersten G (2004) Stability of mono- and trivalent meningococcal outer membrane vesicle vaccines. Vaccine 22(5–6):629–642. https://doi.org/10.1016/j.vaccine.2003.08.027
Arntzen M, Várnai A, Mackie R, Eijsink V, Pope P (2017) Outer membrane vesicles from Fibrobacter succinogenes S85 contain an array of carbohydrate-active enzymes with versatile polysaccharide-degrading capacity. Environ Microbiol 19(7):2701–2714. https://doi.org/10.1111/1462-2920.13770
Aytar-Çelik P, Derkuş B, Erdoğan K, Barut D, Blaise E, Yıldırım Y, Pecha S, Çabuk A (2022) Bacterial membrane vesicle functions, laboratory methods, and applications. Biotechnol Adv 54:107869. https://doi.org/10.1016/j.biotechadv.2021.107869
Baeza N, Mercade E (2020) Relationship between membrane vesicles, extracellular ATP and biofilm formation in Antarctic Gram-Negative bacteria. Microb Ecol 81(3):645–656. https://doi.org/10.1007/S00248-020-01614-6
Baidya AK, Bhattacharya S, Dubey GP, Mamou G, Ben-Yehuda S (2018) Bacterial nanotubes: a conduit for intercellular molecular trade. Curr Opin Microbiol 42:1–6. https://doi.org/10.1016/j.mib.2017.08.006
Balhuizen MD, Veldhuizen EJ, Haagsman HP (2021a) Outer membrane vesicle induction and isolation for vaccine development. Front Microbiol 12:629090. https://doi.org/10.3389/fmicb.2021.629090
Balhuizen MD, Versluis CM, van Harten RM, de Jonge EF, Brouwers JF, van de Lest CHA, Veldhuizen EJA, Tommassen J, Haagsman HP (2021b) PMAP-36 reduces the innate immune response induced by Bordetella bronchiseptica-derived outer membrane vesicles. Curr Res Microb Sci 2:100010. https://doi.org/10.1016/j.crmicr.2020.100010
Baumgarten T, Sperling S, Seifert J, von Bergen M, Steiniger F, Wick LY, Heipieper HJ (2012) Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl Environ Microbiol 78(17):6217–6224. https://doi.org/10.1128/AEM.01525-12
Begić M, Josić D (2020) Biofilm formation and extracellular microvesicles—the way of foodborne pathogens toward resistance. Electrophoresis 41(20):1718–1739. https://doi.org/10.1002/ELPS.202000106
Bernadac A, Gavioli M, Lazzaroni JC, Raina S, Lloubès R (1998) Escherichia coli tol-pal mutants form outer membrane vesicles. J Bacteriol 180(18):4872–4878. https://doi.org/10.1128/JB.180.18.4872-4878.1998
Bitto NJ, Kaparakis-Liaskos M (2017) The therapeutic benefit of bacterial membrane vesicles. Int J Mol Sci 18(6):1–15. https://doi.org/10.3390/ijms18061287
Bitto NJ, Chapman R, Pidot S, Costin A, Lo C, Choi J, D’Cruze T, Reynolds EC, Dashper SG, Turnbull L, Whitchurch CB, Stinear TP, Stacey KJ, Ferrero RL (2017) Bacterial membrane vesicles transport their DNA cargo into host cells. Sci Rep 7(1):1–11. https://doi.org/10.1038/s41598-017-07288-4
Bitto NJ, Zavan L, Johnston EL, Stinear TP, Hill AF, Kaparakis-Liaskos M (2021) Considerations for the analysis of bacterial membrane vesicles: methods of vesicle production and quantification can influence. Microbiol Spectr 9(3):e01273-21. https://doi.org/10.1128/Spectrum.01273-21
Bonnington K, Kuehn M (2014) Protein selection and export via outer membrane vesicles. Biochim Biophys Acta, Mol Cell Res 1843(8):1612–1619. https://doi.org/10.1016/j.bbamcr.2013.12.011
Bonnington K, Kuehn M (2016) Outer membrane vesicle production facilitates LPS remodeling and outer membrane maintenance in Salmonella during environmental transitions. MBio. https://doi.org/10.1128/mBio.01532-16
Bos J, Cisneros LH, Mazel D (2021) Real-time tracking of bacterial membrane vesicles reveals enhanced membrane traffic upon antibiotic exposure. Sci Adv 7(4):1–12. https://doi.org/10.1126/sciadv.abd1033
Briaud P, Carroll RK (2020) Extracellular vesicle biogenesis and functions in gram-positive bacteria. Infect Immun 88(12):1–14. https://doi.org/10.1128/IAI.00433-20
Brown L, Wolf JM, Prados-Rosales R, Casadevall A (2015) Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 3(10):620–630. https://doi.org/10.1038/nrmicro3480
Cañas MA, Giménez R, Fábrega MJ, Toloza L, Baldomà L, Badia J (2016) Outer membrane vesicles from the probiotic Escherichia coli Nissle 1917 and the commensal ECOR12 enter intestinal epithelial cells via clathrin-dependent endocytosis and elicit differential effects on DNA damage. PLoS ONE 11(8):1–22. https://doi.org/10.1371/journal.pone.0160374
Cao Y, Lin H (2021) Characterization and function of membrane vesicles in gram-positive bacteria. Appl Microbiol Biotechnol 105(5):1795–1801. https://doi.org/10.1007/s00253-021-11140-1
Cao Y, Zhou Y, Chen D, Wu R, Guo L, Lin H (2020) Proteomic and metabolic characterization of membrane vesicles derived from Streptococcus mutans at different pH values. Appl Microbiol Biotechnol 104(22):9733–9748. https://doi.org/10.1007/s00253-020-10563-6
Caruana JC, Walper SA (2020) Bacterial membrane vesicles as mediators of microbe—microbe and microbe—host community interactions. Front Microbiol 11:1–24. https://doi.org/10.3389/fmicb.2020.00432
Carvalho AL, Fonseca S, Miquel-Clopés A, Cross K, Kok KS, Wegmann U, Gil-Cordoso K, Bentley EG, Al Katy SHM, Coombes JL, Kipar A, Stentz R, Stewart JP, Carding SR (2019) Bioengineering commensal bacteria-derived outer membrane vesicles for delivery of biologics to the gastrointestinal and respiratory tract. J Extracell Vesicles. https://doi.org/10.1080/20013078.2019.1632100
Chen Q, Bai H, Wu W, Huang G, Li Y, Wu M, Tang G, Ping Y (2020a) Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett 20(1):11–21. https://doi.org/10.1021/acs.nanolett.9b02182
Chen C, Kawamoto J, Kawai S, Tame A, Kato C, Imai T, Kurihara T (2020b) Isolation of a novel bacterial strain capable of producing abundant extracellular membrane vesicles carrying a single major cargo protein and analysis of its transport mechanism. Front Microbiol 10:3001. https://doi.org/10.3389/fmicb.2019.03001
Choi H, Kim M, Jeon J, Han J, Kim K (2017) Overexpression of MicA induces production of OmpC-enriched outer membrane vesicles that protect against Salmonella challenge. Biochem Biophys Res Commun 490(3):991–996. https://doi.org/10.1016/J.BBRC.2017.06.152
Choi J, Kim YK, Han PL (2019) Extracellular vesicles derived from Lactobacillus plantarum increase BDNF expression in cultured hippocampal neurons and produce antidepressant-like effects in mice. Exp Neurobiol 28(2):158–171. https://doi.org/10.5607/en.2019.28.2.158
Choi JH, Moon CM, Shin TS, Kim EK, McDowell A, Jo MK, Joo YH, Kim SE, Jung HK, Shim KN, Jung SA, Kim YK (2020) Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp Mol Med 52(3):423–437. https://doi.org/10.1038/s12276-019-0359-3
Coelho C, Brown L, Maryam M, Vij R, Smith DFQ, Burnet MC, Kyle JE, Heyman HM, Ramirez J, Prados-Rosales R, Lauvau G, Nakayasu ES, Brady NR, Hamacher-Brady A, Coppens I, Casadevall A (2019) Listeria monocytogenes virulence factors, including listeriolysin O, are secreted in biologically active extracellular vesicles. J Biol Chem 294(4):1202–1217. https://doi.org/10.1074/JBC.RA118.006472
De Jonge EF, Balhuizen MD, Van Boxtel R, Wu J, Haagsman HP, Tommassen J (2021) Heat shock enhances outer-membrane vesicle release in Bordetella spp. Curr Res Microb Sci 2:100009. https://doi.org/10.1016/j.crmicr.2020.100009
Dean SN, Rimmer MA, Turner KB, Phillips DA, Caruana JC, Hervey WJ, Leary DH, Walper SA (2020) Lactobacillus acidophilus membrane vesicles as a vehicle of bacteriocin delivery. Front Microbiol 11:1–14
Deatherage BL, Lara JC, Bergsbaken T, Barrett SLR, Lara S, Cookson BT (2009) Biogenesis of bacterial membrane vesicles. Mol Microbiol 72(6):1395–1407. https://doi.org/10.1111/J.1365-2958.2009.06731.X
Dell’Annunziata F, Dell’Aversana C, Doti N, Donadio G, Piaz FD, Izzo V, Filippis A, Galdiero M, Altucci L, Boccia G, Galdiero M, Folliero V, Franci G (2021) Outer membrane vesicles derived from Klebsiella pneumoniae are a driving force for horizontal gene transfer. Int J Mol Sci. https://doi.org/10.3390/ijms22168732
Di Benedetto R, Alfini R, Carducci M, Aruta MG, Lanzilao L, Acquaviva A, Palmieri E, Giannelli C, Necchi F, Saul A, Micoli F (2021) Novel simple conjugation chemistries for decoration of gmma with heterologous antigens. Int J Mol Sci. https://doi.org/10.3390/ijms221910180
Eberlein C, Baumgarten T, Starke S, Heipieper HJ (2018) Immediate response mechanisms of gram-negative solvent-tolerant bacteria to cope with environmental stress: cis-trans isomerization of unsaturated fatty acids and outer membrane vesicle secretion. Appl Microbiol Biotechnol 102(6):2583–2593. https://doi.org/10.1007/s00253-018-8832-9
Elhenawy W, Debelyy MO, Feldman MF (2014) Preferential packing of acidic glycosidases and proteases into bacteroides outer membrane vesicles. mBio. https://doi.org/10.1128/mbio.00909-14.10.1128/mbio.00909-14
Elhenawy W, Bording-Jorgensen M, Valguarnera E, Haurat MF, Wine E, Feldman M (2016) LPS remodeling triggers formation of outer membrane vesicles in Salmonella. Mbio 7(4):940–956. https://doi.org/10.1128/mBio.00940-16
Ellis TN, Kuehn MJ (2010) Virulence and immunomodulatory roles of bacterial outer membrane vesicles. MMBR 74(1):81–94. https://doi.org/10.1128/MMBR.00031-09
Ellis TN, Leiman SA, Kuehn MJ (2010) Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect Immun 78(9):3822–3831. https://doi.org/10.1128/IAI.00433-10
Ezraty B, Gennaris A, Barras F, Collet JF (2017) Oxidative stress, protein damage, and repair in bacteria. Nat Rev Microbiol 15(7):385–396. https://doi.org/10.1038/nrmicro.2017.26
Fantappiè L, de Santis M, Chiarot E, Carboni F, Bensi G, Jousson O, Margarit I, Grandi G (2014) Antibody-mediated immunity induced by engineered Escherichia coli OMVs carrying heterologous antigens in their lumen. J Extracell Vesicles. https://doi.org/10.3402/jev.v3.24015
Florez C, Raab J, Cooke A, Schertzer J (2017) Membrane distribution of the pseudomonas quinolone signal modulates outer membrane vesicle production in Pseudomonas aeruginosa. Bio. https://doi.org/10.1128/MBIO.01034-17
Fulsundar S, Harms K, Flaten GE, Johnsen PJ, Chopade BA, Nielsen KM (2014) Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl Environ Microbiol 80(11):3469–3483. https://doi.org/10.1128/AEM.04248-13
Gamalier JP, Silva TP, Zarantonello V, Dias FF, Melo RCN (2017) Increased production of outer membrane vesicles by cultured freshwater bacteria in response to ultraviolet radiation. Microbiol Res 194:38–46. https://doi.org/10.1016/j.micres.2016.08.002
García-Manrique P, Matos M, Gutiérrez G, Pazos C, Blanco-López MC (2018) Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J Extracell Vesicles. https://doi.org/10.1080/20013078.2017.1422676
Gerke C, Colucci AM, Giannelli C, Sanzone S, Vitali CG, Sollai L, Rossi O, Martin LB, Auerbach J, Di Cioccio V, Saul A (2015) Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS ONE 10(8):e0134478. https://doi.org/10.1371/journal.pone.0134478
Gerritzen MJH, Maas RHW, Van Den Ijssel J, Van Keulen L, Martens DE, Wijffels RH, Stork M (2018) High dissolved oxygen tension triggers outer membrane vesicle formation by Neisseria meningitidis. Microb Cell Fact 17(1):1–10. https://doi.org/10.1186/s12934-018-1007-7
Gerritzen MJH, Martens DE, Uittenbogaard JP, Wijffels RH, Stork M (2019a) Sulfate depletion triggers overproduction of phospholipids and the release of outer membrane vesicles by Neisseria meningitidis. Sci Rep 9(1):1–9. https://doi.org/10.1038/s41598-019-41233-x
Gerritzen MJH, Stangowez L, van de Waterbeemd B, Martens DE, Wijffels RH, Stork M (2019b) Continuous production of Neisseria meningitidis outer membrane vesicles. Appl Microbiol Biotechnol 103(23–24):9401–9410. https://doi.org/10.1007/s00253-019-10163-z
Giacomucci S, Mathieu-Denoncourt A, Vincent AT, Jannadi H, Duperthuy M (2022) Experimental evolution of Vibrio cholerae identifies hypervesiculation as a way to increase motility in the presence of polymyxin B. Front Microbiol 13:3143. https://doi.org/10.3389/FMICB.2022.932165/BIBTEX
Gill S, Catchpole R, Forterre P (2019) Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol Rev 43(3):273–303. https://doi.org/10.1093/femsre/fuy042
Gnopo YMD, Watkins HC, Stevenson TC, DeLisa MP, Putnam D (2017) Designer outer membrane vesicles as immunomodulatory systems—reprogramming bacteria for vaccine delivery. Adv Drug Deliv Rev 114:132–142. https://doi.org/10.1016/j.addr.2017.05.003
Godlewska R, Klim J, Debski J, Wyszynska A, Lasica A (2019) Influence of environmental and genetic factors on proteomic profiling of outer membrane vesicles. Pol J Microbiol 68(2):255–261
Goreham RV, Ayed Z, Ayupova D, Dobhal G (2019) Extracellular vesicles: nature’s own nanoparticles. In: Andrews DL, Lipson RH, Nann T (eds) Comprehensive Nanoscience and Nanotechnology, 2nd edn. Academic Press, pp 27–48
Gorringe AR, Pajon R (2012) Bexsero: A multicomponent vaccine for prevention of meningococcal disease. Hum Vaccines Immunother 8(2):174–183. https://doi.org/10.4161/hv.18500
Grandi A, Fantappiè L, Irene C, Valensin S, Tomasi M, Stupia S, Corbellari R, Caproni E, Zanella I, Isaac SJ, Ganfini L, Frattini L, König E, Gagliardi A, Tavarini S, Sammicheli C, Parri M, Grandi G (2018) Vaccination With a FAT1-derived B cell epitope combined with tumor-specific B and T cell epitopes elicits additive protection in cancer mouse models. Front Oncol 8:481. https://doi.org/10.3389/fonc.2018.00481
Guan N, Li J, Shin HD, Du G, Chen J, Liu L (2017) Microbial response to environmental stresses: from fundamental mechanisms to practical applications. Appl Microbiol Biotechnol 101(10):3991–4008. https://doi.org/10.1007/s00253-017-8264-y
Guerrero-Mandujano A, Hernández-Cortez C, Ibarra JA, Castro-Escarpulli G (2017) The outer membrane vesicles: secretion system type zero. Traffic 18(7):425–432. https://doi.org/10.1111/TRA.12488
Gurung M, Moon DC, Choi CW, Lee JH, Bae YC, Kim J, Lee YC, Seol SY, Cho DT, Kim S, Lee JC (2011) Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS ONE. https://doi.org/10.1371/journal.pone.0027958
Gujrati V, Kim S, Kim SH, Min JJ, Choy HE, Kim SC, Jon S (2014) Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano 8(2):1525–1537. https://doi.org/10.1021/nn405724x
Hahm J, Kim J, Park J (2021) Strategies to enhance extracellular vesicle production. Tissue Eng Regen Med 18(4):513–524. https://doi.org/10.1007/s13770-021-00364-x
Harisa GI, Sherif AY, Youssof AME, Alanazi FK, Salem-Bekhit MM (2020) Bacteriosomes as a promising tool in biomedical applications: immunotherapy and drug delivery. AAPS PharmSciTech 21(5):1–13. https://doi.org/10.1208/s12249-020-01716-x
Hayashi J, Hamada N, Kuramitsu HK (2002) The autolysin of Porphyromonas gingivalis is involved in outer membrane vesicle release. FEMS Microbio Lett 216(2):217–222. https://doi.org/10.1111/J.1574-6968.2002.TB11438.X
Herrmann IK, Wood MJA, Fuhrmann G (2021) Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol 16(7):748–759. https://doi.org/10.1038/s41565-021-00931-2
Hews CL, Cho T, Rowley G, Raivio TL (2019) Maintaining integrity under stress: envelope stress response regulation of pathogenesis in gram-negative bacteria. Front Cell Infect Microbiol 9:313. https://doi.org/10.3389/fcimb.2019.00313
Hirayama S, Nakao R (2020) Glycine significantly enhances bacterial membrane vesicle production: a powerful approach for isolation of LPS-reduced membrane vesicles of probiotic Escherichia coli. Microb Biotechnol 13(4):1162–1178. https://doi.org/10.1111/1751-7915.13572
Holst J, Oster P, Arnold R, Tatley MV, Næss LM, Aaberge IS, Galloway Y, McNicholas A, O’Hallahan J, Rosenqvist E, Black S (2013) Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum Vaccin Immunother 9(6):1241–1253. https://doi.org/10.4161/hv.24129
Hozbor D, Rodriguez ME, Fernández J, Lagares A, Guiso N, Yantorno O (1999) Release of outer membrane vesicles from Bordetella pertussis. Curr Microbiol 38(5):273–278. https://doi.org/10.1007/PL00006801
Hu R, Li J, Zhao Y, Lin H, Liang L, Wang M, Liu H, Min Y, Gao Y, Yang M (2020) Exploiting bacterial outer membrane vesicles as a cross-protective vaccine candidate against avian pathogenic Escherichia coli (APEC). Microb Cell Fact 19(1):1–17. https://doi.org/10.1186/s12934-020-01372-7
Hu K, Palmieri E, Samnuan K, Ricchetti B, Oldrini D, McKay PF, Wu G, Thorne L, Fooks AR, McElhinney LM, Goharriz H, Golding M, Shattock RJ, Micoli F (2022) Generalized modules for membrane antigens (GMMA), an outer membrane vesicle-based vaccine platform, for efficient viral antigen delivery. J Extracell Vesicles. https://doi.org/10.1002/jev2.12247
Hua L, Yang Z, Li W, Zhang Q, Ren Z, Ye C, Zheng X, Li D, Long Q, Bai H, Sun W, Yang X, Zheng P, He J, Chen Y, Huang W, Ma Y (2021) A novel immunomodulator delivery platform based on bacterial biomimetic vesicles for enhanced antitumor immunity. Adv Mater 33(43):1–18. https://doi.org/10.1002/adma.202103923
Huang W, Wang S, Yao Y, Xia Y, Yang X, Li K, Sun P, Liu C, Sun W, Bai H, Chu X, Li Y, Ma Y (2016) Employing Escherichia coli - derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against Acinetobacter baumannii infection. Sci Rep 6:37242. https://doi.org/10.1038/srep37242
Huang W, Meng L, Chen Y, Dong Z, Peng Q (2022a) Bacterial outer membrane vesicles as potential biological nanomaterials for antibacterial therapy. Acta Biomater 140:102–115. https://doi.org/10.1016/j.actbio.2021.12.005
Huang Y, Nieh MP, Chen W, Lei Y (2022b) Outer membrane vesicles (OMVs) enabled bio-applications: a critical review. Biotechnol Bioeng 119(1):34–47. https://doi.org/10.1002/bit.27965
Jahromi LP, Fuhrmann G (2021) Bacterial extracellular vesicles: understanding biology promotes applications as nanopharmaceuticals. Adv Drug Deliv Rev 173:125–140. https://doi.org/10.1016/j.addr.2021.03.012
Jan AT (2017) Outer Membrane Vesicles (OMVs) of gram-negative bacteria: a perspective update. Front Microbiol 8:1–11. https://doi.org/10.3389/fmicb.2017.01053
Jeong D, Kim MJ, Park Y, Chung J, Kweon HS, Kang NG, Hwang SJ, Youn SH, Hwang BK, Kim D (2022) Visualizing extracellular vesicle biogenesis in gram-positive bacteria using super-resolution microscopy. BMC Biol 20(1):1–14. https://doi.org/10.1186/s12915-022-01472-3
Jiang L, Driedonks TAP, Jong WSP, Dhakal S, van den Berg B, van Saparoea H, Sitaras I, Zhou R, Caputo C, Littlefield K, Lowman M, Chen M, Lima G, Gololobova O, Smith B, Mahairaki V, Riley Richardson M, Mulka KR, Lane AP, Klein SL, Pekosz A, Brayton C, Mankowski JL, Luirink J, Villano JS, Witwer KW (2022) A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and Delta variants. J Extracell Vesicles 11(3):e12192. https://doi.org/10.1002/JEV2.12192
Joffe LS, Nimrichter L, Rodrigues ML, Del Poeta M (2016) Potential roles of fungal extracellular vesicles during infection. Msphere 1(4):11–14. https://doi.org/10.1128/msphere.00099-16
Kaparakis-Liaskos M, Ferrero RL (2015) Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol 15(6):375–387. https://doi.org/10.1038/nri3837
Keiser PB, Gibbs BT, Coster TS, Moran EE, Stoddard MB, Labrie JE 3rd, Schmiel DH, Pinto V, Chen P, Zollinger WD (2010) A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine 28(43):6970–6976. https://doi.org/10.1016/j.vaccine.2010.08.048
Kesty NC, Kuehn MJ (2004) Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J Biol Chem 279(3):2069–2076. https://doi.org/10.1074/jbc.M307628200
Kim JH, Lee J, Park J, Gho YS (2015a) Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol 40:97–104. https://doi.org/10.1016/j.semcdb.2015.02.006
Kim OY, Choi SJ, Jang SC, Park KS, Kim SR, Choi JP, Lim JH, Lee SW, Park J, Di Vizio D, Lötvall J, Kim YK, Gho YS (2015b) Bacterial protoplast-derived nanovesicles as a vaccine delivery system against bacterial infection. Nano Lett 15(1):266–274. https://doi.org/10.1021/nl503508h
Kim H, Kim M, Myoung K, Kim W, Ko J, Kim KP, Cho EG (2020a) Comparative lipidomic analysis of extracellular vesicles derived from Lactobacillus plantarum apsulloc 331261 living in green tea leaves using liquid chromatography-mass spectrometry. Int J Mol Sci 21(21):1–19. https://doi.org/10.3390/ijms21218076
Kim SW, Seo JS, Park SB, Lee AR, Lee JS, Jung JW, Chun JH, Lazarte JMS, Kim J, Kim JH, Song JW, Franco C, Zhang W, Ha MW, Paek SM, Jung M, Jung TS (2020b) Significant increase in the secretion of extracellular vesicles and antibiotics resistance from methicillin-resistant Staphylococcus aureus induced by ampicillin stress. Sci Rep 10(1):1–14. https://doi.org/10.1038/s41598-020-78121-8
Klieve AV, Yokoyama MT, Forster RJ, Ouwerkerk D, Bain PA, Mawhinney EL (2005) Naturally occurring DNA transfer system associated with membrane vesicles in cellulolytic Ruminococcus spp. of ruminal origin. Appl Environ Microbiol 71(8):4248–4253. https://doi.org/10.1128/AEM.71.8.4248-4253.2005
Klimentova J, Pavkova I, Horcickova L, Bavlovic J, Kofronova O, Benada O, Stulik J (2019) Francisella tularensis subsp. holarctica releases differentially loaded outer membrane vesicles under various stress conditions. Front Microbiol 10:1–16. https://doi.org/10.3389/fmicb.2019.02304
Klimentová J, Stulík J (2015) Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol Res 170:1–9. https://doi.org/10.1016/j.micres.2014.09.006
Kobayashi H, Uematsu K, Hirayama H, Horikoshi K (2000) Novel toluene elimination system in a toluene-tolerant microorganism. J Bacteriol 182(22):6451–6455
Koning RI, de Breij A, Oostergetel GT, Nibbering PH, Koster AJ, Dijkshoorn L (2013) Cryo-electron tomography analysis of membrane vesicles from Acinetobacter baumannii ATCC19606T. Res Microbiol 164(5):397–405. https://doi.org/10.1016/j.resmic.2013.02.007
Kovacs SA, Titball RW, Michell SL (2011) Lipoproteins of bacterial pathogens. Infect Immun 79(2):548–561
Laloux G, Collet JF (2017) Major Tom to ground control: how lipoproteins communicate extracytoplasmic stress to the decision center of the cell. J Bacteriol 199(21):1–13. https://doi.org/10.1128/JB.00216-17
Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W (2010) The bacterial ghost platform system: production and applications. Bioeng Bugs 1(5):326–336. https://doi.org/10.4161/BBUG.1.5.12540
Lappann M, Otto A, Becher D, Vogel U (2013) Comparative proteome analysis of spontaneous outer membrane vesicles and purified outer membranes of Neisseria meningitidis. J Bacteriol 195(19):4425–4435. https://doi.org/10.1128/JB.00625-13
Launay O, Lewis DJM, Anemona A, Loulergue P, Leahy J, Sciré AS, Maugard A, Marchetti E, Zancan S, Huo Z, Rondini S, Marhaba R, Finco O, Martin LB, Auerbach J, Cohen D, Saul A, Gerke C, Podda A (2017) Safety profile and immunologic responses of a novel vaccine against shigella sonnei administered intramuscularly, intradermally and intranasally: results from two parallel randomized phase 1 clinical studies in healthy adult volunteers in Europe. EBioMedicine 22:164–172. https://doi.org/10.1016/J.EBIOM.2017.07.013
Lee BH, Wu SC, Shen TL, Hsu YY, Chen CH, Hsu WH (2021) The applications of Lactobacillus plantarum-derived extracellular vesicles as a novel natural antibacterial agent for improving quality and safety in tuna fish. Food Chem 340:128104. https://doi.org/10.1016/j.foodchem.2020.128104
Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS (2009) Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9(24):5425–5436. https://doi.org/10.1002/PMIC.200900338
Lee J, Lee EY, Kim SH, Kim DK, Park KS, Kim KP, Kim YK, Roh TY, Gho YS (2013) Staphylococcus aureus extracellular vesicles carry biologically active β-lactamase. Antimicrob Agents Chemother 57(6):2589–2595. https://doi.org/10.1128/AAC.00522-12
Lee T, Jun SH, Choi CW, Il KS, Lee JC, Shin JH (2018) Salt stress affects global protein expression profiles of extracellular membrane-derived vesicles of Listeria monocytogenes. Microb Pathog 115:272–279. https://doi.org/10.1016/j.micpath.2017.12.071
Li M, Zhou H, Yang C, Wu Y, Zhou X, Liu H, Wang Y (2020) Bacterial outer membrane vesicles as a platform for biomedical applications: an update. J Control Release 323:253–268. https://doi.org/10.1016/j.jconrel.2020.04.031
Li W, Hu Y, Zhang Q, Hua L, Yang Z, Ren Z, Zheng X, Huang W, Ma Y (2021) Development of drug-resistant Klebsiella pneumoniae vaccine via novel vesicle production technology. AC S Appl Mater Interfaces 13(28):32703–32715. https://doi.org/10.1021/acsami.1c06701
Li Y, Wu J, Qiu X, Dong S, He J, Liu J, Xu W, Huang S, Hu X, Xiang DX (2023) Bacterial outer membrane vesicles-based therapeutic platform eradicates triple-negative breast tumor by combinational photodynamic/chemo-/immunotherapy. Bioact Mater 20:548–560. https://doi.org/10.1016/j.bioactmat.2022.05.037
Liao S, Klein MI, Heim KP, Fan Y, Bitoun JP, Ahn SJ, Burne RA, Koo H, Brady LJ, Wen ZT (2014) Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J Bacteriol 196(13):2355–2366. https://doi.org/10.1128/JB.01493-14
Lin J, Zhang W, Cheng J, Yang X, Zhu K, Wang Y, Wei G, Qian PY, Luo ZQ, Shen X (2017) A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat Commun 8(1):1–12. https://doi.org/10.1038/ncomms14888
Liu Y, Defourny KAY, Smid EJ, Abee T (2018) Gram-positive bacterial extracellular vesicles and their impact on health and disease. Front Microbiol 9:1–8. https://doi.org/10.3389/fmicb.2018.01502
Liu Y, Tempelaars MH, Boeren S, Alexeeva S, Smid EJ, Abee T (2022a) Extracellular vesicle formation in Lactococcus lactis is stimulated by prophage-encoded holin–lysin system. Microb Biotechnol 15(4):1281–1295. https://doi.org/10.1111/1751-7915.13972
Liu H, Geng Z, Su J (2022b) Engineered mammalian and bacterial extracellular vesicles as promising nanocarriers for targeted therapy. Evcna 3(1):63–86
Liu H, Zhang Q, Wang S, Weng W, Jing Y, Su J (2022c) Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: advances and perspectives. Bioact Mater 14:169–181. https://doi.org/10.1016/j.bioactmat.2021.12.006
Liu H, Li M, Zhang T, Liu X, Zhang H, Geng Z, Su J (2022d) Engineered bacterial extracellular vesicles for osteoporosis therapy. Chem Eng J 450:138309. https://doi.org/10.1016/J.CEJ.2022.138309
Lu G, Jiang X, Wu A, Zhou J, Liu H, He F, Zhang Q, Zen K, Gu S, Wang J (2021) Two small extracellular vesicle sRNAs derived from Mycobacterium tuberculosis serve as diagnostic biomarkers for active pulmonary tuberculosis. Front Microbiol. https://doi.org/10.3389/FMICB.2021.642559
MacDonald IA, Kuehn MJ (2013) Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J Bacteriol 195(13):2971–2981. https://doi.org/10.1128/JB.02267-12
Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E (2014) Unleashing the potential of NOD- and toll-like agonists as vaccine adjuvants. PNAS 111(34):12294–12299. https://doi.org/10.1073/pnas.1400478111
Malinverni JC, Silhavy TJ (2009) An ABC transport system that maintains lipid asymmetry in the Gram-negative outer membrane. PNAS 106(19):8009–8014. https://doi.org/10.1073/pnas.0903229106
Mancini F, Rossi O, Necchi F, Micoli F (2020) OMV Vaccines and the role of TLR agonists in immune response. Int J Mol Sci 21(12):4416. https://doi.org/10.3390/ijms21124416
Mancini F, Micoli F, Necchi F, Pizza M, Berlanda SF, Rossi O (2021) GMMA-Based vaccines: the known and the unknown. Front Immunol 12:715393. https://doi.org/10.3389/fimmu.2021.715393
Manning AJ, Kuehn MJ (2011) Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol 11:258. https://doi.org/10.1186/1471-2180-11-258
Maharjan S, Saleem M, Feavers IM, Wheeler JX, Care R, Derrick JP (2016) Dissection of the function of the RmpM periplasmic protein from Neisseria meningitidis. Microbiology 162(2):364–375. https://doi.org/10.1099/mic.0.000227
Mathelié-Guinlet M, Asmar AT, Collet JF, Dufrêne YF (2020) Lipoprotein Lpp regulates the mechanical properties of the E. coli cell envelope. Nat Commun 11:1789. https://doi.org/10.1038/s41467-020-15489-1
McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ (2006) Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol 188(15):5385–5392. https://doi.org/10.1128/JB.00498-06
McBroom AJ, Kuehn MJ (2007) Release of outer membrane vesicles by gram-negative bacteria is a novel envelope stress response. Mol Microbiol 63(2):545–558. https://doi.org/10.1111/j.1365-2958.2006.05522.x
McCaig WD, Loving CL, Hughes HR, Brockmeier SL (2016) Characterization and vaccine potential of outer membrane vesicles produced by Haemophilus parasuis. PLoS ONE 11(3):1–23. https://doi.org/10.1371/journal.pone.0149132
McMahon KJ, Castelli ME, Vescovi EG, Feldman MF (2012) Biogenesis of outer membrane vesicles in Serratia marcescens is thermoregulated and can be induced by activation of the Rcs phosphorelay system. J Bacteriol 194(12):3241–3249. https://doi.org/10.1128/JB.00016-12
McMillan HM, Zebell SG, Ristaino JB, Dong X, Kuehn MJ (2021) Protective plant immune responses are elicited by bacterial outer membrane vesicles. Cell Rep 34(3):108645. https://doi.org/10.1016/j.celrep.2020.108645
Michel LV, Gallardo L, Konovalova A, Bauer M, Jackson N, Zavorin M, McNamara C, Pierce J, Cheng S, Snyder E, Hellman J, Pichichero ME (2020) Ampicillin triggers the release of Pal in toxic vesicles from Escherichia coli. Int J Antimicrob Agents 56(6):106163. https://doi.org/10.1016/j.ijantimicag.2020.106163
Micoli F, MacLennan CA (2020) Outer membrane vesicle vaccines. Semin Immunol 50:101433. https://doi.org/10.1016/j.smim.2020.101433
Micoli F, Alfini R, Di Benedetto R, Necchi F, Schiavo F, Mancini F, Carducci M, Oldrini D, Pitirollo O, Gasperini G, Balocchi C, Bechi N, Brunelli B, Piccioli D, Adamo R (2021) Generalized modules for membrane antigens as carrier for polysaccharides: impact of sugar length, density, and attachment site on the immune response elicited in animal models. Front Immunol 12:719315. https://doi.org/10.3389/fimmu.2021.719315
Morishita M, Horita M, Higuchi A, Marui M, Katsumi H, Yamamoto A (2021) Characterizing different probiotic-derived extracellular vesicles as a novel adjuvant for immunotherapy. Mol Pharm 18(3):1080–1092. https://doi.org/10.1021/acs.molpharmaceut.0c01011
Mougenot MF, Pereira VS, Costa ALR, Lancellotti M, Porcionatto MA, da Silveira JC, de la Torre LG (2022) Biomimetic nanovesicles—sources, design, production methods, and applications. Pharmaceutics 14(10):1–25. https://doi.org/10.3390/pharmaceutics14102008
Mozaheb N, Mingeot-Leclercq MP (2020) Membrane vesicle production as a bacterial defense against stress. Front Microbiol 11:600221. https://doi.org/10.3389/fmicb.2020.600221
Muralinath M, Kuehn MJ, Roland KL, Curtiss R (2011) Immunization with Salmonella enterica serovar Typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae. Infect Immun 79(2):887–894. https://doi.org/10.1128/IAI.00950-10
Nagakubo T, Nomura N, Toyofuku M (2020) Cracking open bacterial membrane vesicles. Front Microbiol 10:3026. https://doi.org/10.3389/fmicb.2019.03026
Ñahui RA, Vanpouille C, Costantini PE, Margolis L (2021) Microbiota–host communications: bacterial extracellular vesicles as a common language. PLoS Pathog 17(5):1–20. https://doi.org/10.1371/journal.ppat.1009508
Nevermann J, Silva A, Otero C, Oyarzún DP, Barrera B, Gil F, Calderón IL, Fuentes JA (2019) Identification of genes involved in biogenesis of Outer Membrane Vesicles (OMVs) in Salmonella enterica Serovar Typhi. Front Microbiol 10:1–17. https://doi.org/10.3389/fmicb.2019.00104
Ojima Y, Mohanadas T, Kitamura K, Nunogami S, Yajima R, Taya M (2016) Deletion of degQ gene enhances outer membrane vesicle production of Shewanella oneidensis cells. Arch Microbiol 199(3):415–423. https://doi.org/10.1007/S00203-016-1315-4
Ojima Y, Yamaguchi K, Taya M (2018) Quantitative evaluation of recombinant protein packaged into outer membrane vesicles of Escherichia coli cells. Biotechnol Prog 34(1):51–57. https://doi.org/10.1002/btpr.2536
Ojima Y, Sawabe T, Konami K, Azuma M (2020) Construction of hypervesiculation Escherichia coli strains and application for secretory protein production. Biotechnol Bioeng 117(3):701–709. https://doi.org/10.1002/bit.27239
Ojima Y, Sawabe T, Nakagawa M, Tahara YO, Miyata M, Azuma M (2021) Aberrant membrane structures in hypervesiculating Escherichia coli Strain ΔmlaEΔnlpI visualized by electron microscopy. Front Microbiol 12:706525. https://doi.org/10.3389/fmicb.2021.706525
Palacios A, Coelho C, Maryam M, Luque JL, Casadevall A, Prados R (2020) Biogenesis and function of extracellular vesicles in gram-positive bacteria, mycobacteria, and fungi. In: Kaparakis M, Kufer TA (eds) Bacterial Membrane Vesicles: Biogenesis, Functions and Applications. Springer, Cham, pp 47–74
Park K, Svennerholm K, Crescitelli R, Lässer C, Gribonika I, Lötvall J (2021) Synthetic bacterial vesicles combined with tumour extracellular vesicles as cancer immunotherapy. J Extracell Vesicles. https://doi.org/10.1002/jev2.12120
Pasqua M, Zennaro A, Trirocco R, Fanelli G, Micheli G, Grossi M, Colonna B, Prosseda G (2021) Modulation of omv production by the lysis module of the dlp12 defective prophage of Escherichia coli k12. Microorganisms 9(2):1–14. https://doi.org/10.3390/microorganisms9020369
Pathirana RD, Kaparakis-Liaskos M (2016) Bacterial membrane vesicles: biogenesis, immune regulation and pathogenesis. Cell Microbiol 18(11):1518–1524. https://doi.org/10.1111/cmi.12658
Peng LH, Wang MZ, Chu Y, Zhang L, Niu J, Shao HT, Yuan TJ, Jiang ZH, Gao JQ, Ning XH (2020) Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL-programmed therapy against melanoma. Sci Adv. https://doi.org/10.1126/sciadv.aba2735
Pérez-Cruz C, Delgado L, López-Iglesias C, Mercade E (2015) Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria. PLoS ONE 10(1):1–18. https://doi.org/10.1371/journal.pone.0116896
Pérez-Cruz C, Cañas MA, GiménezR BJ, Mercade E, Baldomà L, Aguilera L (2016) Membrane vesicles released by a hypervesiculating Escherichia coli Nissle 1917 tolR mutant are highly heterogeneous and show reduced capacity for epithelial cell interaction and entry. PLoS ONE 11(12):1–20. https://doi.org/10.1371/journal.pone.0169186
Prados-Rosales R, Baena A, Martinez LR, Luque-Garcia J, Kalscheuer R, Veeraraghavan U, Camara C, Nosanchuk JD, Besra GS, Chen B, Jimenez J, Glatman-Freedman A, Jacobs WR, Porcelli SA, Casadevall A (2011) Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. JCI 121(4):1471–1483. https://doi.org/10.1172/JCI44261
Premjani V, Tilley D, Gruenheid S, Le Moual H, Samis JA (2014) Enterohemorrhagic Escherichia coli OmpT regulates outer membrane vesicle biogenesis. FEMS Microbiol Lett 355(2):185–192. https://doi.org/10.1111/1574-6968.12463
Reimer SL, Beniac DR, Hiebert SL, Booth TF, Chong PM, Westmacott GR, Zhanel GG, Bay DC (2021) Comparative analysis of outer membrane vesicle isolation methods with an Escherichia coli tolA mutant reveals a hypervesiculating phenotype with outer-inner membrane vesicle content. Front Microbiol 12:1–17. https://doi.org/10.3389/fmicb.2021.628801
Remis JP, Wei D, Gorur A, Zemla M, Haraga J, Allen S, Witkowska HE, Costerton JW, Berleman JE, Auer M (2014) Bacterial social networks: structure and composition of Myxococcus xanthus outer membrane vesicle chains. Environ Microbiol 16(2):598–610. https://doi.org/10.1111/1462-2920.12187
Richter M, Vader P, Fuhrmann G (2021a) Approaches to surface engineering of extracellular vesicles. Adv Drug Deliv Rev 173:416–426. https://doi.org/10.1016/j.addr.2021.03.020
Richter R, Kamal MAM, Koch M, Niebuur B, Huber A, Goes A, Volz C, Vergalli J, Kraus T, Müller R, Schneider-Daum N, Fuhrmann G, Pagès J, Lehr C (2021b) An outer membrane vesicle-based permeation assay (OMPA) for assessing bacterial bioavailability. Adv Healthc Mater. https://doi.org/10.1002/adhm.202101180
Roier S, Zingl FG, Cakar F, Durakovic S, Kohl P, Eichmann TO, Klug L, Gadermaier B, Weinzerl K, Prassl R, Lass A, Daum G, Reidl J, Feldman MF, Schild S (2016) A novel mechanism for the biogenesis of outer membrane vesicles in gram-negative bacteria. Nat Commun 7(6):257–259. https://doi.org/10.1038/ncomms10515
Schaar V, Nordström T, Mörgelin M, Riesbeck K (2011) Moraxella catarrhalis outer membrane vesicles carry β-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob Agents Chemother 55(8):3845–3853. https://doi.org/10.1128/AAC.01772-10
Schulz E, Goes A, Garcia R, Panter F, Koch M, Müller R, Fuhrmann K, Fuhrmann G (2018) Biocompatible bacteria-derived vesicles show inherent antimicrobial activity. J Control Release 290:46–55. https://doi.org/10.1016/j.jconrel.2018.09.030
Schwechheimer C, Kuehn MJ (2013) Synthetic effect between envelope stress and lack of outer membrane vesicle production in Escherichia coli. J Bacteriol 195(18):4161–4173. https://doi.org/10.1128/JB.02192-12
Schwechheimer C, Kuehn MJ (2015) Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol 13(10):605–619
Schwechheimer C, Sullivan C, Kuehn M (2013) Envelope control of outer membrane vesicle production in gram-negative bacteria. Biochem 52(18):3031–3040. https://doi.org/10.1021/BI400164T
Schwechheimer C, Kulp A, Kuehn MJ (2014) Modulation of bacterial outer membrane vesicle production by envelope structure and content. BMC Microbiol. https://doi.org/10.1186/s12866-014-0324-1
Schwechheimer C, Rodriguez DL, Kuehn MJ (2015) NlpI-mediated modulation of outer membrane vesicle production through peptidoglycan dynamics in Escherichia coli. Microbiol Open 4(3):375–389. https://doi.org/10.1002/mbo3.244
Seo MK, Park EJ, Ko SY, Choi EW, Kim S (2018) Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2,4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. JDS 101(10):8662–8671. https://doi.org/10.3168/jds.2018-15014
Shetty A, Hickey WJ (2014) Effects of outer membrane vesicle formation, surface-layer production and nanopod development on the metabolism of phenanthrene by Delftia acidovorans Cs1-4. PLoS ONE 9(3):1–6. https://doi.org/10.1371/journal.pone.0092143
Shi Y, Meng L, Zhang C, Zhang F, Fang Y (2022) Extracellular vesicles of Lacticaseibacillus paracasei PC-H1 induce colorectal cancer cells apoptosis via PDK1/AKT/Bcl-2 signaling pathway. Microbiol Res 255:126921. https://doi.org/10.1016/J.MICRES.2021.126921
Song T, Mika F, Lindmark B, Liu Z, Schild S, Bishop A, Zhu J, Camilli A, Johansson J, Vogel J, Wai S (2008) A new Vibrio cholerae sRNA modulates colonization and affects release of outer membrane vesicles. Mol Microbiol 70(1):100–111. https://doi.org/10.1111/J.1365-2958.2008.06392.X
Song JW, Baeg Y, Jeong HY, Lee J, Oh DK, Hollmann F, Park JB (2021) Bacterial outer membrane vesicles as nano-scale bioreactors: a fatty acid conversion case study. ChemCatChem 13(19):4080–4086. https://doi.org/10.1002/cctc.202100778
Stentz R, Osborne S, Horn N, Li AWH, Hautefort I, Bongaerts R, Rouyer M, Bailey P, Shears SB, Hemmings AM, Brearley CA, Carding SR (2014) A bacterial homolog of a eukaryotic inositol phosphate signaling enzyme mediates cross-kingdom dialog in the mammalian gut. Cell Rep 6(4):646–656. https://doi.org/10.1016/J.CELREP.2014.01.021
Takaki K, Tahara YO, Nakamichi N, Hasegawa Y, Shintani M, Ohkuma M, Miyata M, Futamata H, Tashiro Y (2020) Multilamellar and multivesicular outer membrane vesicles produced by a Buttiauxella agrestis tolB Mutant. Appl Environ Microbiol 86(20):1–15. https://doi.org/10.1128/AEM.01131-20
Tashiro Y, Yawata Y, Toyofuku M, Uchiyama H, Nomura N (2013) Interspecies interaction between Pseudomonas aeruginosa and other microorganisms. Microbes Environ 28(1):13–24. https://doi.org/10.1264/JSME2.ME12167
Thoma J, Manioglu S, Kalbermatter D, Bosshart PD, Fotiadis D, Müller DJ (2018) Protein-enriched outer membrane vesicles as a native platform for outer membrane protein studies. Commun Biol. https://doi.org/10.1038/s42003-018-0027-5
Thomas SC, Madaan T, Kamble NS, Siddiqui NA, Pauletti GM, Kotagiri N (2022) Engineered bacteria enhance immunotherapy and targeted therapy through stromal remodeling of tumors. Adv Healthc Mater 11(2):1–15. https://doi.org/10.1002/adhm.202101487
Toyofuku M, Zhou S, Sawada I, Takaya N, Hiroo U, Nomura N (2014) Membrane vesicle formation is associated with pyocin production under denitrifying conditions in Pseudomonas aeruginosa PAO1. Environ Microbiol 16:2927–2938. https://doi.org/10.1111/1462-2920.12260
Toyofuku M, Cárcamo-Oyarce G, Yamamoto T, Eisenstein F, Hsiao CC, Kurosawa M, Gademann K, Pilhofer M, Nomura N, Eberl L (2017) Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat Commun 8(1):1–10. https://doi.org/10.1038/s41467-017-00492-w
Toyofuku M, Nomura N, Eberl L (2019) Types and origins of bacterial membrane vesicles. Nat Rev Microbiol 17(1):13–24. https://doi.org/10.1038/s41579-018-0112-2
Turnbull L, Toyofuku M, Hynen AL, Kurosawa M, Pessi G, Petty NK, Osvath SR, Cárcamo-Oyarce G, Gloag ES, Shimoni R, Omasits U, Ito S, Yap X, Monahan LG, Cavaliere R, Ahrens CH, Charles IG, Nomura N, Eberl L, Whitchurch CB (2016) Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun 7:11220. https://doi.org/10.1038/ncomms11220
Turner L, Praszkier J, Hutton ML, Steer D, Ramm G, Kaparakis-Liaskos M, Ferrero RL (2015) Increased outer membrane vesicle formation in a Helicobacter pylori tolB Mutant. Helicobacter 20(4):269–283. https://doi.org/10.1111/hel.12196
Uddin MJ, Dawan J, Jeon G, Yu T, He X, Ahn J (2020) The role of bacterial membrane vesicles in the dissemination of antibiotic resistance and as promising carriers for therapeutic agent delivery. Microorganisms 8(5):1–23. https://doi.org/10.3390/microorganisms8050670
van der Pol L, Stork M, van der Ley P (2015) Outer membrane vesicles as platform vaccine technology. Biotechnol J 10(11):1689–1706. https://doi.org/10.1002/biot.201400395
van de Waterbeemd B, Streefland M, van der Ley P, Zomer B, van Dijken H, Martens D, Wijffels R, van der Pol LA (2010) Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccine 28(30):4810–4816. https://doi.org/10.1016/j.vaccine.2010.04.082
van de Waterbeemd B, Streefland M, van Keulen L, van den IJssel J, de Haan A, Eppink MH, van der Pol LA (2012) Identification and optimization of critical process parameters for the production of NOMV vaccine against Neisseria meningitidis. Vaccine 30(24):3683–3690. https://doi.org/10.1016/j.vaccine.2012.03.028
van de Waterbeemd B, Zomer G, Kaaijk P, Ruiterkamp N, Wijffels RH, van den Dobbelsteen GPJM, van der Pol LA (2013a) Improved production process for native outer membrane vesicle vaccine against Neisseria meningitidis. PLoS ONE 8(5):1–11. https://doi.org/10.1371/journal.pone.0065157
van de Waterbeemd B, Zomer G, van den Ijssel J, van Keulen L, Eppink MH, van der Ley P, van der Pol LA (2013b) Cysteine depletion causes oxidative stress and triggers outer membrane vesicle release by Neisseria meningitidis, implications for vaccine development. PLoS ONE. https://doi.org/10.1371/journal.pone.0054314
Wang W, Chanda W, Zhong M (2015) The relationship between biofilm and outer membrane vesicles: a novel therapy overview. FEMS Microbiol Lett 362(15):117. https://doi.org/10.1093/FEMSLE/FNV117
Wang X, Thompson C, Weidenmaier C, Lee J (2018) Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat Commun. https://doi.org/10.1038/s41467-018-03847-z
Wang X, Eagen W, Lee J (2020) Orchestration of human macrophage NLRP3 inflammasome activation by Staphylococcus aureus extracellular vesicles. PNAS 117(6):3174–3184. https://doi.org/10.1073/pnas.1915829117
Wang X, Koffi PF, English OF, Lee JC (2021a) Staphylococcus aureus extracellular vesicles: a story of toxicity and the stress of 2020. Toxins 13:75. https://doi.org/10.3390/toxins13020075
Wang Y, Hoffmann JP, Baker SM, Höner K, Wimley WC, Fuselier JA, Bitoun JP, Morici LA (2021b) Inhibition of Streptococcus mutans biofilms with bacterial-derived outer membrane vesicles. BMC Microbiol 21(234):1–12. https://doi.org/10.1186/s12866-021-02296-x
Wei S, Jiao D, Xing W (2022) A rapid method for isolation of bacterial extracellular vesicles from culture media using epsilon-poly-L–lysine that enables immunological function research. Front Immunol 13:1–13. https://doi.org/10.3389/fimmu.2022.930510
Wolf JM, Rivera J, Casadevall A (2012) Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles. Cell Microbiol 14(5):762–773. https://doi.org/10.1111/J.1462-5822.2012.01757.X
Wyke A, Ward JB, Hayes M, Curtis N (1981) A role in vivo for penicillin-binding protein-4 of Staphylococcus aureus. Eur J Biochem 119(2):389–393. https://doi.org/10.1111/J.1432-1033.1981.TB05620.X
Yamasaki-Yashiki S, Sakamoto Y, Nishimura K, Saika A, Ito T, Kunisawa J, Katakura Y (2024) High productivity of immunostimulatory membrane vesicles of using glycine. Bmfh 43(1):55–63
Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-Da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:1–60. https://doi.org/10.3402/jev.v4.27066
Yokoyama F, Imai T, Aoki W, Ueda M, Kawamoto J, Kurihara T (2021) Identification of a putative sensor protein involved in regulation of vesicle production by a hypervesiculating bacterium, Shewanella vesiculosa HM13. Front Microbiol 12:1–13. https://doi.org/10.3389/fmicb.2021.629023
Youssof AME, Alanazi FK, Salem-Bekhit MM, Shakeel F, Haq N (2019) Bacterial ghosts carrying 5-fluorouracil: a novel biological carrier for targeting colorectal cancer. AAPS PharmSciTech. https://doi.org/10.1208/S12249-018-1249-Z
Zanella I, König E, Tomasi M, Gagliardi A, Frattini L, Fantappiè L, Irene C, Zerbini F, Caproni E, Isaac SJ, Grigolato M, Corbellari R, Valensin S, Ferlenghi I, Giusti F, Bini L, Ashhab Y, Grandi A, Grandi G (2021) Proteome-minimized outer membrane vesicles from Escherichia coli as a generalized vaccine platform. J Extracell Vesicles. https://doi.org/10.1002/jev2.12066
Zarantonello V, Silva TP, Noyma NP, Gamalier JP, Mello MM, Marinho MM, Melo RCN (2018) The Cyanobacterium Cylindrospermopsis raciborskii (CYRF-01) responds to environmental stresses with increased vesiculation detected at single-cell resolution. Front Microbiol 9:272. https://doi.org/10.3389/fmicb.2018.00272
Zhang Y, Fang Z, Li R, Huang X, Liu Q (2019a) Design of outer membrane vesicles as cancer vaccines: a new toolkit for cancer therapy. Cancers 11(9):1314. https://doi.org/10.3390/cancers11091314
Zhang Y, Chen Y, Lo C, Zhuang J, Angsantikul P, Zhang Q, Wei X, Zhou Z, Obonyo M, Fang RH, Gao W, Zhang L (2019b) Inhibition of pathogen adhesion by bacterial outer membrane-coated nanoparticles. Angew. Chem. Int. Ed. Engl 58(33):11404–11408. https://doi.org/10.1002/ANIE.201906280
Zingl FG, Leitner DR, Schild S (2020) Biogenesis of gram-negative. In: Kaparakis-Liaskos M, Kufer TA (eds) Bacterial membrane vesicles: biogenesis, functions and applications, 1st edn. Springer, Cham, pp 23–46
Zlatkov N, Nadeem A, Uhlin BE, Wai SN (2021) Eco-evolutionary feedbacks mediated by bacterial membrane vesicles. FEMS Microbiol Rev 45(2):1–26. https://doi.org/10.1093/femsre/fuaa047
Acknowledgements
LMM-E is a doctoral student from Programa de Doctorado en Ciencias Biológicas, UNAM, and receive a fellowship from “Consejo Nacional de Humanidades, Ciencias y Tecnologías” CONAHCYT (CVU 1102921). SB-L is a doctoral student from Doctorado en Ciencias Biomédicas, UNAM, and receive a fellowship from “Consejo Nacional de Humanidades, Ciencias y Tecnologías” CONAHCYT (CVU 1102922). NAV-C and MAT-R thank the sabbatical to “Programa de Apoyos para la Superación del Personal Académico” PASPA-DGAPA- UNAM. The authors thank Dr. Lucia Perezgasga, Dr. Rafael Vazquez-Duhalt, M. Sc. Rentería Maciel Monserrat, M. Comm. Sonia Olguín García, M. Sc. Itandehui Betanzo, and Dr. Oscar González-Davis for their technical and scientific support. This project was developed under the Institutional Program of the Instituto de Investigaciones Biomédicas UNAM: “La producción de biomoléculas de interés biomédico en bacterias y hongos”.
Funding
Open access funding provided by Universidad Nacional Autónoma de México through a Transformative Agreement. This work was supported by “Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, Universidad Nacional Autónoma de México” (PAPIIT-UNAM, IN210822: NAVC, IN211422, IV201220: MATR) and Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT, CF-2023-I-1248 and CF-2023-I-1549). The funders had no role in the decision to publish or prepare the manuscript.
Author information
Authors and Affiliations
Contributions
The manuscript has been designed and compiled by LMM-E and NAV-C. LMM-E and NAV-C have prepared the table and figures. All authors have contributed equally towards the drafting and revising of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muñoz-Echeverri, L.M., Benavides-López, S., Geiger, O. et al. Bacterial extracellular vesicles: biotechnological perspective for enhanced productivity. World J Microbiol Biotechnol 40, 174 (2024). https://doi.org/10.1007/s11274-024-03963-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-024-03963-7