Abstract
Saikosaponin d (SSd) is an important bioactive compound of traditional Chinese medicinal plant Bupleurum scorzonerifolium Willd. and exhibits many effects, such as anti-tumor, anti-inflammation and immunomodulatory. Since endophytic fungi possess the natural capacity to produce the similar secondary metabolite to that of their host plants, they are promising as alternative sources of plant bioactive natural products. In this study, in order to search for SSd-producing strains, endophytes were isolated from B. scorzonerifolium and were authenticated by the ITS sequence and the translation elongation factor-1alpha gene (TEF-1α) sequence analysis. The profile of metabolites present in the crude exacts was carried out by ultra performance liquid chromatography time-of-flight mass spectrometry (UPLC/Q-TOF-MS) analysis. The results showed that two strains, CHS2 and CHS3 from B. scorzonerifolium could produce SSd by UPLC/Q-TOF-MS analysis, and the amount of SSd produced by strain CHS2 and CHS3 were about 2.17 and 2.40 µg/mL, respectively. CHS2 and CHS3 showed a close phylogenetic relationship to Fusarium oxysporum and Fusarium acuminatum, respectively. According to our concern, no endophytic fungi capable of producing SSd from B. scorzonerifolium have been found before. Our clear intention was to isolate and identify these endophytic fungi that produce important active secondary metabolites, and then study the strains that produce this compound on a large scale through fermentation or even genetic study, to provide a feasible and more convenient way for the production of SSd.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epiphytic and endophytic are group of microorganisms that live in healthy host plants (Santamaría and Bayman 2005). Endophytes generally exist in various tissues and organs inside the host plants as least a part of their life cycle without directly causing any obvious external diseases and adverse results (Stone et al. 2000; Quilliam and Jones 2010). It is known that endophytes are beneficial to hosts by protecting plants against a variety of biotic and abiotic stresses (Yan et al. 2019; Abeer et al. 2016; Bian et al. 2021). Endophytic fungi possess the natural capacity to produce and accumulate secondary metabolites. These secondary metabolites have various biological activities and can be directly or indirectly used as medicine to treat different diseases (Aly et al. 2010; Nisa et al. 2015; Kusari and Spiteller 2012; Staniek et al. 2008). In recent years, the metabolites from endophytic microorganisms have shown that endophytes have been valued as potential sources of anti-cancer, antioxidant, antibiotics and immunosuppressive bioactive compounds (Kamel et al. 2020). Endophytic fungi are regarded as alternative resources of medicinal plants because they can produce the same bioactive compounds as their host plant, such as alkaloids, terpenoids, steroids and phenols.
Nan-Chai-Hu, also named Radix Bupleuri, the root of Bupleurum scorzonerifolium Willd. (Umbelliferae), is a traditional Chinese herb which is used in treating of tumor, allergy and inflammation in Asia (Ashour et al. 2009; The Pharmacopoeia Commission of the PRC 2015). This plant’s secondary metabolites might offer us with a variety of active compounds containing flavonoids, essential oils, coumarins, lignans, triterpene saponins, polyacetylenes and alkaloids. These bioactive components are crucial in disease treatments (Ashour and Wink 2011). Among them, saikosaponins (SSs) were found to be the most active compounds, especially saikosaponin d (SSd), which exhibits many effects, such as anti-tumor, anti-inflammation and immunomodulatory (Zhou et al. 2021; Li et al. 2018). In our previous work, the endophytic fungi from B. scorzonerifolium were isolated and the distribution characteristics of endophytes in B. scorzonerifolium have been investigated (Gao et al. 2012).
The central goal of this study was to screen the endophytic fungi with the capability of SSd producing from B. scorzonerifolium. Ultra performance liquid chromatography time-of-flight mass spectrometry (UPLC/Q-TOF-MS) analysis was performed to detect whether the endophytes isolated from B. scorzonerifolium could produce the similar compounds to host plants. Up to now, no endophytic fungi of SSd producing from B. scorzonerifolium have been reported.
Materials and methods
Isolation of endophytes from B. scorzonerifolium
Endophytic fungi were isolated from different parts (roots, stems, leaves and flowers) of B. scorzonerifolium with aseptic operation. For surface sterilization, each part of the plant was washed with sterile water, followed by soaking in 75% ethanol (v/v) for 3 min, rinsing twice with sterile water, soaking in 0.1% mercuric chloride (v/v) for 5 min, and rinsing with sterile water for three times. 5 × 5 mm of segments cut from the treated plant material were cultured on potato dextrose agar (PDA) medium (1 L PDA medium containing potato 200 g, dextrose 20 g and agar 12 g) at 25 °C, checking the growth of endophytic fungi in plant segments every day.
Ethanol extraction from endophytic fungi
The endophytic fungi were cultured in potato dextrose broth (PDB) medium (1 L PDB medium containing potato 200 g and dextrose 20 g) at 28 °C and shaking at 160 rpm for 10 days in the dark. The cultured fungi were collected by vacuum filtration, and then grinded into powder in liquid nitrogen. Ethanol extraction from the fungal powder with 70% ethanol was evaporated with a rotary evaporator (Eyela, Jpn). The extractions were prepared for SSd analysis by UPLC/Q-TOF-MS.
SSd analysis by UPLC/Q-TOF-MS
The Waters ACQUITY™ UPLC system (Waters Corporation, MA, USA) was used for UPLC–MS analysis of the samples. The C18 reversed-phase column (ACQUITY UPLC™ BEH, 50 mm × 2.1 mm, i.d., 1.7 μm) was used and kept at 40 °C. The liquid chromatography was equipped a 190–400 nm detector. 4 µL of sample was injected. The flow rate was set to 400 µL/min and gradient mobile phase (solvent A was 0.1% formic acid in demonized water, solvent B was 0.1% formic acid in acetonitrile) which was programmed as follows: 0 min, 2% B; 1.0 min, 10% B; 4.0 min, 10% B; 12.0 min, 70% B; 14.0 min, 98% B; 15.0 min, 2% B and kept at 2% B for 2 min, given a total running time of 17 min.
The sample is analyzed by Water Xevo quadrupole time-of-flight mass spectrometer (MS; Manchester, UK), and its metabolic spectrum needs to be collected by an electrospray ionization (ESI) source. According to the preliminary experiment of the system determination, the spectral determination was carried out in the positive ion mode, and the optimum parameters are as follows: desolvation temperature of 350 °C, source temperature of 120 °C, cone voltage of 25 V and capillary voltage of 3.0 kV. Nitrogen with flow rate of 650 L/h and 50 L/h was used as nitrogen and conical gas respectively. The data acquisition rate of the instrument was adjusted to 0.2 s and the scanning interval delay is 0.02 s. The scanning range was 100–1000 m/z. In order to ensure the accuracy and reproducibility of the data, all analyses were done through the use of locking spray. Leucine–enkephalin was used as the positive ion model of acetonitrile (0.1% formic acid):H2O (0.1% formic acid) with a concentration of 400 pg/mL ([M + H] + = 556.2771). The Pareto mode was used to collect the data, and the frequency of phase-locked spray was set to 1 s. Before using the Mass Lynx 4.1 software to process the data, the average scanning of the phase-locked quality data was more than 10 times for correction.
DNA extraction and fungal identification
The mycelia of the two strains were inoculated into the flask containing 200 mL of PDB medium, and the mycelium biomass of about 100 mg was harvested after incubation on the shaker (160 rpm, 28 °C, 3 days). According to the research of Guo et al. (2001), the genomic DNA of endophytic fungi was isolated and extracted by the CTAB method.
Fungal identification was based on their ITS sequences and TEF-1α sequences, which was accomplished by PCR amplification with the universal primers (V9D 5′-TTA AGT CCC TGC CCT TTG TA-3′; LS266 5′-GCA TTC CCA AAC AAC TCG ACT C-3′) and TEF-1α primers (TEF1-728 F 5′-CAT CGA GAA GTT CGA GAA GG-3′; TEF1-rev 5′-GCC ATC CTT GGA GAT ACC AGC-3′) (Van Den Gerrits and Hoog 1999; Carbone and Kohn 1999). Reaction volumes of 50 µL contained 1 mM dNTPs, 1.75 units of Taq DNA polymerase, 0.2 µg of genomic DNA, 1.5 mM MgCl2, 5 µL 10 × PCR buffer and 20 pM of each primer. The mixed samples were amplified in Bio-RadT100TM Thermal Cycler (Bio-Rad, USA) to obtain PCR products. The 5 µl PCR product was verified by electrophoresis display on 1% (w/v) agarose gel in 1× TAE buffer (1 mmoL/L EDTA, 40 mmoL/L Tris, pH 8.0). Then the same primers were used for sequencing (Shanghai Sangon Biologic Engineering Technology and Service Co., Ltd., Shanghai, People's Republic of China).
Phylogenetic analysis
The BLAST algorithm was used to compare the corresponding ITS sequence and TEF-1α sequence of each strains with the NCBI (National Center for Biotechnology Information) data set on GenBank (NCBI; http://www.ncbi.nlm.nih.gov). The DNAMAN program was used to perform multiple sequence alignments. Molecular evolutionary analysis and the phylogenetic tree construction were conducted using neighbor-joining method by MEGA version 11.0 (Kumar et al. 2008).
Results
To assay endophytic fungal metabolites with a reasonable elution time, isocratic elution of acetonitrile–water was performed as described in the experimental section. In our work, the metabolites of CHS3 and CHS2 (isolated from the stems of B. scorzonerifolium) with a running time of 17 min showed the same peaks as those of the SSd standards when eluted at 11.08 and 11.12 min, respectively. The metabolites of CHS2 have the same cleavage fragments as the SSD standard at m/z 149.0204, 455.3502, 763.4695 (Fig. 1). The metabolites of CHS3 regularly show several fragments at m/z 149.0189, 437.3424, 455.3536, 601.4117 and 763.4784, which are the same as the cleavage fragments of SSd standard (Fig. 1). The amount of SSd produced by CHS2 and CHS3 were 2.17 and 2.40 µg/mL under the condition described in this study. After subculture, the two endophytic fungi still had a stable ability to produce SSd, and the strains were stored in degree cryogenic refrigerator at − 80 °C.
To further determine the phylogeny of strain CHS2 and CHS3, the ITS1-5.8 S-ITS2 sequences and TEF-1α sequence of these isolates were amplified and sequenced (Fig. 2). Then the sequences were compared to corresponding sequences of referenced fungal taxa in the database.
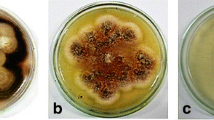
Also, these fungi were identified by morphological characteristics (Fig. 3). CHS2 grew rapidly in PDA medium, the colony was nearly round, the edge was neat, the colony was reddish in the middle, the edge was white, and the texture was loose and fluffy. According to the observation of mycelial microscope, the conidium was irregular and the conidia were chain-shaped. According to the morphological characteristics, CHS2 was preliminarily identified as Fusarium sp. (Fig. 3). CHS3 grew rapidly on PDA medium, the colony was round and uniform, and the hyphae were white, fluffy and well developed. Under microscope, the branches of conidiophores were irregular and distributed in groups. Thick-walled spores are formed in the peduncle of hyphae and cysts. According to the morphological characteristics, CHS3 was preliminarily identified as Fusarium sp. (Fig. 3).
Based on ITS sequence and TEF-1α sequence, homologous sequence search with GenBank showed that the similarity between CHS2 sequence and Fusarium oxysporum was 95% and 98% (GenBank accession numbers KC119203.1 and MT886217.1), and that between CHS3 sequence and Fusarium acuminatum was 99% and 97% (GenBank accession numbers HM068320.1 and MF523228.1) (Table 1). The phylogenetic relationship was established by comparing and branching the homologous nucleotide sequences of CHS2 and CHS3 in GenBank. According to the phylogenetic analysis, the isolates of CHS2 and CHS3 were classified as F. oxysporum and F. acuminatum, respectively (Figs. 4, 5).
Identities were obtained by blasting sequences in NCBI
Discussion
Like host plants, endophytic fungi can also possess the natural capacity to produce bioactive secondary metabolites with the same or similar chemical structures (Lamshöft et al. 2008; Cui et al. 2012; Silvia et al. 2007). Therefore, endophytic fungi are considered as potential microbial resources to replace traditional medicinal plants to produce pharmacologically active natural compounds, and have very broad medicinal value and research prospects (Aly et al. 2011). Traditional medicinal plants are the top-priority for isolation and screening of endophytic fungi (Gómez and Luiz 2018). The discovery of SSd-producing endophytic fungi is valuable for industrial application. The production of SSd by endophytes CHS2 and CHS3 further supports the theory that through long-term symbiotic relationships between endophytic fungi and plants, endophytes may acquire genes from their host plants and could synthesize analogous or identical bioactive secondary metabolites to their hosts. This indicated that CHS3 and CHS2 could be substituted for B. scorzonerifolium to produce SSd in the fermentation industry.
The genus Fusarium is widely distributed and is an important source of secondary metabolites, such as camptothecin and vincristine, so it has been widely concerned by people and has become the focus of research in the last few years (Kusari et al. 2009a, b). According to our concern, the genus Fusarium has not been found to have the ability to produce SSd before. In this paper, we first reported two SSd-producing endophytic fungi isolated from B. scorzonerifolium.
However, the SSd production by each of the two endophytes in axenic cultures is too low to match expectation. Because endophytes always interact with hosts and other endophytes, the biosynthesis ability of endophytic fungi in aseptic culture is very different from that in host plants. When observed metabolic production of the endophytic fungus isolated from Camptotheca acuminate, through the first to the seventh-generation subculture, Kusari et al. found that a sharp attenuation occurred in the production of camptothecin by this endophyte (Kusari et al. 2009a, b). They believe that the lack of host stimulation in aseptic cultures may be one of the reasons for the attenuation. It is highly probable that there might be metabolic communication between the endophytes and the host plants, and in vivo metabolic processes of endophytes are likely regulated by plants. Though the mechanisms by which endophytes interact with host plants are incompletely understood, we agreed with the theory that in the process of evolution, endophytic fungi have developed mechanisms for biosynthesis and tolerance to high levels of secondary metabolites in order to better compete and survive with medicinal plants (Kusari et al. 2012). The study of interspecific crosstalk between endophyte–endophyte and endophyte–host is worthy of further study. We have established endophytic–endophytic and endophytic–host co-culture models, and are currently studying the ways in which fungi and hosts interact to promote SSd production.
Conclusion
This is the first time that we have screened two endophytic fungi with stable genetic characters and the ability to produce SSd. CHS2 and CHS3 were identified as F. oxysporum and F. acuminatum respectively by ITS sequence and TEF-1α sequence analysis. All these efforts further prove the natural ability of endophytes to produce the same or similar bioactive substances as host plants, and provide a reliable and stable source for such secondary metabolites in the future.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Abeer H, Abd Allah EF, Alqarawi AA, Al-Huqail AA, Stephan W, Dilfuza E (2016) The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front Microbiol 7:1089. https://doi.org/10.3389/fmicb.2016.01089
Aly AH, Debbab A, Kjer J, Proksch P (2010) Fungal endophytes from higher plants: a prolific source of phytochemicals and other bioactive natural products. Fungal Divers 41:1–16. https://doi.org/10.1016/j.micpath.2015.04.001
Aly AH, Debbab A, Proksch P (2011) Fungal endophytes: unique plant inhabitants with great promises. Appl Microbiol Biotechnol 90:1829–1845. https://doi.org/10.1007/s00253-011-3270-y
Ashour ML, Wink M (2011) Genus Bupleurum: a review of its phytochemistry, pharmacology and modes of action. J Pharm Pharmacol 63:305–321. https://doi.org/10.1111/j.2042-7158.2010.01170.x
Ashour ML, El-Readi M, Youns M, Mulyaningsih S, Sporer F, Efferth T, Wink M (2009) Chemical composition and biological activity of the essential oil obtained from Bupleurum marginatum (Apiaceae). J Pharm Pharmacol 61:1–9. https://doi.org/10.1211/jpp/61.08.0012
Bian JY, Fang YL, Song Q, Sun ML, Yang JY, Ju YW, Li DW, Huang L (2021) The fungal endophyte Epicoccum dendrobii as a potential biocontrol agent against Colletotrichum gloeosporioides. Phytopathology 111(2):293–303. https://doi.org/10.1094/PHYTO-05-20-0170-R
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in 13 filamentous Ascomycetes. Mycologia 91(3):553–556
Cui Y, Yi D, Bai X, Sun B, Zhao Y, Zhang Y (2012) Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia 83(5):913–920. https://doi.org/10.1016/j.fitote.2012.04.009
Gao N, Wang Q, Wang ZY, Cheng YP (2012) Distribution characteristics of endophytic fungi from Bupleurum scorzonerifolium Willd. For Byprod Spec China 1:10–11 (in Chinese)
Gómez OC, Luiz J (2018) Endophytic fungi isolated from medicinal plants: future prospects of bioactive natural products from Tabebuia/Handroanthus endophytes. Appl Microbiol Biotechnol 102(21):9105–9119. https://doi.org/10.1007/s00253-018-9344-3
Guo LD, Hyde KD, Liew EC (2001) Detection and taxonomic placement of endophytic fungi within frond tissues of Livistona chinensis based on rDNA sequences. Mol Phylogenet Evol 20:1–13. https://doi.org/10.1006/mpev.2001.0942
Kamel NM, Abdel-Motaal FF, El-Zayat SA (2020) Endophytic fungi from the medicinal herb Euphorbia geniculata as a potential source for bioactive metabolites. Arch Microbiol 202(2):247–255
Kumar S, Dudley J, Nei M, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9:299–306. https://doi.org/10.1093/bib/bbn017
Kusari S, Spiteller M (2012) Metabolomics of endophytic fungi producing associated plant secondary metabolites: progress, challenges and opportunities. In: Roessner U (ed) Metabolomics. InTech, Rijeka, pp 241–266
Kusari S, Lamshöft M, Spiteller M (2009a) Aspergillus fumigates Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J Appl Microbiol 10:1019–1030. https://doi.org/10.1111/j.1365-2672.2009.04285.x
Kusari S, Zühlke S, Spiteller M (2009b) An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J Nat Prod 72:2–7. https://doi.org/10.1021/np800455b
Kusari S, Hertweck C, Spiteller M (2012) Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem Biol 19:792–798. https://doi.org/10.1016/j.chembiol.2012.06.004
Lamshöft M, Zühlke S, Spiteller M (2008) An endophytic fungus from Hypericum perforatum that produces hypericin. J Nat Prod 71:159–162. https://doi.org/10.1021/np070669k
Li XQ, Song YN, Wang SJ, Rahman K, Zhu JY, Zhang H (2018) Saikosaponins: a review of pharmacological effects. J Asian Nat Prod Res 20(5):399–411. https://doi.org/10.1080/10286020.2018.1465937
Nisa H, Kamili AN, Nawchoo IA, Shafi S, Shameem N, Bandh SA (2015) Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: a review. Microb Pathog 82:50–59. https://doi.org/10.1016/j.micpath.2015.04.001
Quilliam RS, Jones DL (2010) Fungal root endophytes of the carnivorous plant Drosera rotundifolia. Mycorrhiza 20:341–348. https://doi.org/10.1007/s00572-009-0288-4
Santamaría J, Bayman P (2005) Fungal epiphytes and endophytes of coffee leave (Coffea arabica). Microb Ecol 50:1–8. https://doi.org/10.1007/s00248-004-0002-1
Silvia F, Sturdikova M, Muckova M (2007) Bioactive secondary metabolites produced by microorganisms associated with plants. Biologia 62:251–257
Staniek A, Woerdenbag HJ, Kayser O (2008) Endophytes: exploiting biodiversity for the improvement of natural product-based drug discovery. J Plant Interact 3:75–93
Stone JK, Bacon CW, White JF Jr (2000) An overview of endophytic microbes: endophytism defined. In: Bacon CW, White JF Jr (eds) Microbial endophytes. Marcel Dekker, New York, pp 3–30
The Pharmacopoeia Commission of the PRC (2015) Pharmacopoeia of People’s Republic of China. Chemical Industry Press, Beijing, pp 263–264
Van Den Gerrits AHG, Hoog GS (1999) Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Stud Mycol 43:151–162
Yan L, Zhu J, Zhao X, Shi J, Jiang C, Shao D (2019) Beneficial effects of endophytic fungi colonization on plants. Appl Microbiol Biotechnol 103(8):3327–3340. https://doi.org/10.1007/s00253-019-09713-2
Zhou P, Shi W, He XY, Du QY, Wang F, Guo J (2021) Saikosaponin D: review on the antitumour effects, toxicity and pharmacokinetics. Pharm Biol 59(1):1480–1489. https://doi.org/10.1080/13880209.2021.1992448
Funding
This research was financially supported by the NSFC (81573539), Heilongjiang Touyan Innovation Team Program, Natural Science Foundation of Heilongjiang Province (H2015042), Natural Science Foundation of Education Department of Heilongjiang Province (12531621) and Graduate Innovation Program of Heilongjiang University of Chinese Medicine (2022yjscx057).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the concept and design of the study. YC and GL participated in the isolation and SSd analysis of endophytic fungi, ZL and YZ participated in DNA extraction and amplification, and constructed a phylogenetic tree. All the authors participated in the writing of each chapter of the article and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, Y., Liu, G., Li, Z. et al. Screening saikosaponin d (SSd)-producing endophytic fungi from Bupleurum scorzonerifolium Willd. World J Microbiol Biotechnol 38, 242 (2022). https://doi.org/10.1007/s11274-022-03434-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03434-x