Abstract
Peatlands are recognised as an important but vulnerable ecological resource. Understanding the effects of existing damage, in this case erosion, enables more informed land management decisions to be made. Over the growing seasons of 2013 and 2014 photosynthesis and ecosystem respiration were measured using closed chamber techniques within vegetated haggs and erosional peat pans in Dartmoor National Park, southwest England. Below-ground total and heterotrophic respiration were measured and autotrophic respiration estimated from the vegetated haggs. The mean water table was significantly higher in the peat pans than in the vegetated haggs; because of this, and the switching from submerged to dry peat, there were differences in vegetation composition, photosynthesis and ecosystem respiration. In the peat pans photosynthetic CO2 uptake and ecosystem respiration were greater than in the vegetated haggs and strongly dependent on the depth to water table (r2 > 0.78, p < 0.001). Whilst in the vegetated haggs, photosynthesis and ecosystem respiration had the strongest relationships with normalised difference vegetation index (NDVI) (r2 = 0.82, p < 0.001) and soil temperature at 15 cm depth (r2 = 0.77, p = 0.001). Autotrophic and total below-ground respiration in the vegetated haggs varied with soil temperature; heterotrophic respiration increased as water tables fell. An empirically derived net ecosystem model estimated that over the two growing seasons both the vegetated haggs (29 and 20 gC m−2; 95% confidence intervals of − 570 to 762 and − 873 to 1105 gC m−2) and the peat pans (7 and 8 gC m−2; 95% confidence intervals of − 147 to 465 and − 136 to 436 gC m−2) were most likely net CO2 sources. This study suggests that not only the visibly degraded bare peat pans but also the surrounding vegetated haggs are losing carbon to the atmosphere, particularly during warmer and drier conditions, highlighting a need for ecohydrological restoration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peatlands are recognised as valuable ecological resources providing a range of ecosystem services including food provision, flood alleviation, drinking water supply, amenity value and carbon sequestration (Grand-Clement et al. 2013). However, many peatlands are damaged, putting these ecosystem services at risk (Holden et al. 2007). Projects aimed at restoring the ecohydrological functioning of mires are more likely to set realistic targets and succeed where the effects of existing damage are understood (Bonnett et al. 2009).
Blanket bogs form in cool (< 15 °C mean summer temperatures) and wet (> 1000 mm rain annually) conditions (Lindsay et al. 1988). They consist of multiple peat-forming conditions (e.g. raised bogs, watershed mires, flushes etc.) which have spread laterally and joined together smothering the underlying topography. As they rise above the influence of groundwater they are dependent on precipitation for both water and nutrients (Moore 1987) resulting in acidic nutrient poor conditions. In the UK, ecohydrologically functioning blanket bogs are dominated by Sphagnum species which maintain the water table at or above the ground surface (Clymo 1983; Evans et al. 1999; Holden et al. 2011). The UK has 10–15% of the world’s blanket peat resource (Tallis 1997) primarily located in upland areas, consequently they are globally important.
Burning, grazing, deposition of atmospheric pollutants (Yeloff et al. 2006), thawing permafrost (Schuur et al. 2008) and climate change (Stevenson et al. 1990) have all been proposed as causes of peatland erosion. These can initiate a feedback loop where peat erosion reduces vegetation cover leaving bare peat more susceptible to further erosion by fluvial, aeolian and freeze–thaw processes forming erosional features (Bragg and Tallis 2001). These features are of great concern as they drain the peat, resulting in particulate organic carbon losses downstream (Evans et al. 2006) and water table draw-down in the surrounding vegetated areas (Daniels et al. 2008), altering vegetation composition and CO2 fluxes beyond their extent (Clay et al. 2012).
Lower water tables have been shown to alter the vegetation present away from Sphagnum towards vascular plants such as Molinia caerulea, Calluna vulgaris and Eriophorum species (Coulson et al. 1990; Bellamy et al. 2012). These species have larger and more dynamic CO2 fluxes (McNamara et al. 2008; Otieno et al. 2009) but are more readily decomposed (Coulson and Butterfield 1978; Wallen 1993; Thormann et al. 1999) and therefore contribute little to the long-term carbon store compared to Sphagnum. Additionally vascular plants can have extensive root systems which may stimulate the decomposition of more recalcitrant deeper peat (Fontaine et al. 2007).
To date, studies on CO2 fluxes from eroding blanket bogs (McNamara et al. 2008; Clay et al. 2012; Dixon et al. 2013; Rowson et al. 2013) have been focused in northern England where erosional gullies are steeper, deeper and wider (up to 3 m) than the peat pans of Dartmoor (up to 1.5 m wide and typically < 50 cm deep). It is unclear what initiated erosion on Dartmoor but peat pans (shallow, sparsely vegetated, hydraulically-connected, intermittently saturated depressions) and haggs (surrounding vegetated blocks) are limited to flat areas with insufficient erosional energy for gullies to form. They expand as the water level is lowered around the edges of the vegetated haggs destabilising the peat (Luscombe, pers. comm., 2018).
The deep peats of Dartmoor store an estimated 13.1 Mt of carbon (Gatis et al. 2019) but are vulnerable to climate change as they lie at the southern limit of the UK blanket bog climatic envelope (Clark et al. 2010). This makes them invaluable as indicators of the potential effects of climate change on other, more northerly, maritime peatlands. Understanding the controls on CO2 fluxes on Dartmoor may provide an indication of the future for other deep peats as temperatures rise potentially initiating more erosion. The aim of this study was to investigate the controls on CO2 fluxes in peat haggs and peat pans and quantify CO2 fluxes from these landscape components.
Materials and methods
Study site
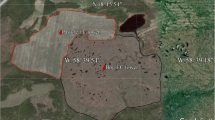
The study site is located in an area of degraded blanket bog in Dartmoor National Park (Fig. 1a and b), southwest England (50°36 N, 3°57′W). At Princetown (Fig. 1b) the long-term average annual precipitation is 1974 mm and has a mean monthly temperature range of 0.8 to 17.7 °C. The site lies at 515 m above sea level and is classified as National Vegetation Classification class M17 Scirpus cespitosus-Eriophorum vaginatum blanket mire (Rodwell 1991). Peat at the study site is estimated to be between 3.6 and 4.0 m thick (Gatis et al. 2019) above the average for Dartmoor (0.81 m) (Parry et al. 2012). The study site is within an extensive area of erosional peat pans and vegetated haggs (Fig. 1c and d). The areas is currently used for extensive sheep grazing.
Location of a Dartmoor National Park (shaded area) within the south west of England, b Study site (red square) within the national park (grey), c the study site (black square) within an area of erosion, and d the arrangement of plots and monitoring equipment; vegetated (grey squares) and bare (white squares) net CO2 ecosystem exchange and total (white circles) and heterotrophic (black circle) below ground respiration. In c and d the green vegetated areas are the vegetated haggs and the grey, watery areas are the peat pans
Net CO2 ecosystem exchange measurements
Net CO2 Ecosystem Exchange (NEE) measurements were taken on 10 separate dates at six locations in the vegetated haggs in a randomised pattern approximately every month between 05/06/2013 and 20/09/2013 and 10/04/2014 and 10/09/2014. A 20 cm diameter, 50 cm tall Perspex collar was attached to the peat surface prior to each measurement using silicon putty (Evo-Stick “Plumbers Mait”, Stafford, UK), and subsequently removed at the end of the measurement. The collar was 50 cm tall to allow for the expected mid-summer vegetation height. The collar was not inserted into the peat as this severs fine surface roots (Heinemeyer et al. 2011) and alters the hydrological and micro-meteorological properties of the peat. Due to limited moss coverage it was possible to ensure a good seal with the peat surface directly using silicon putty. A LiCOR-8100 infra-red gas analyser (LiCOR, Lincoln, Nebraska) connected to a 8100-104C transparent chamber (with a rubber gasket to ensure an airtight seal) measured variation in CO2 concentrations every 2 s over 2 min concurrently with photosynthetic photon flux density (PPFD) (LiCOR Li-190 Quantum Sensor). In order to limit the weight of equipment carried to site it was not possible to control chamber temperature, therefore temperature may have increased during the test potentially stressing the plants resulting in an underestimate of photosynthesis. The variation in chamber temperature over the tests ranged from 0.0 to 1.4 °C. To further limit this effect the chamber was removed between measurements to restore ambient conditions.
In the peat pans CO2 measurements were taken on 15 separate dates at six locations in a randomised pattern approximately every 2 weeks (concurrent with soil respiration measurements) from 29/05/2013 to 07/10/2013 and 14/04/2014 and 11/09/2014. The 8100-104C transparent chamber was too heavy to float so CO2 concentration was measured from a 16 cm diameter, 13 cm tall floating collars every 9 s over 90 s using an EGM-4 infra-red gas analyser and a transparent CPY-4 canopy assimilation chamber (2.427 l) (PP Systems, Hitchin) concurrently with chamber air temperature and PPFD. No ebullition or sudden increases in CO2 suggestive of ebullition were observed so it is assumed CO2 was not released via this mechanism. Measurement of the same location via the two different methods resulted in an uncertainty of 0.2 μmol m−2 s−1.
CO2 measurements were taken on sunny days at 100, 60, 40, 10 and 0% light levels using a combination of shade cloths. It is acknowledged that shade cloths can underestimate photosynthesis at low light levels compared to naturally low light conditions. However, this seemed the most practical solution given the remoteness of the site and labour availability. The net CO2 exchange at each light level was calculated from the linear change in chamber CO2 concentration. Linear accumulation rates with an r2 of less than 0.7 were discarded unless the maximum change in CO2 concentration was ≤ 1 ppm in which case a flux of 0 μmol m−2 s−1 was assigned. Of the 1041 samples collected, 8 were discarded from the vegetated haggs and 25 from the peat pans at this stage.
To account for variability in solar radiation between measurements, net CO2 fluxes were fitted to a hyperbolic light response curve (Eq. 1) using a non-linear least-squares fit across the different light levels measured for each plot for each month.
where NEE is the net CO2 ecosystem exchange (μmol m−2 s−1), Pmax is the rate of light saturated photosynthesis (μmol m−2 s−1), k is the half-saturation constant of photosynthesis (μmol photons m−2 s−1), PPFD the incident photosynthetic photon flux density (PPFD) (μmol Photons m−2 s−1) and REco ecosystem respiration (μmol m−2 s−1). Only light response curves with an r2 > 0.7 were accepted therefore a further 11 and 171 measurements from the vegetated haggs and peat pans respectively were discarded.
REco was determined for each plot from each light response curve (one per sample day) using Eq. 1. Photosynthesis and net ecosystem exchange were then determined for a PPFD of 1000 μmol photons m−2 s−1 (PG1000 and NEE1000), the light saturated photosynthesis rate, using Eq. 1 and the parameters Pmax, and K previously determined from each light response curve. As different PPFD meters were used in the peat pans and vegetated haggs, a cross-calibration with a continuous (every 15 min) onsite global irradiation meter (r2 > 0.90, n = 284; Adcon, Klosterneuburg, Austria) was used to ensure that fluxes were being standardised to the same light intensity.
Soil CO2 efflux measurements
At each vegetated hagg plot four Polyvinyl Chloride collars (16 cm diameter, 8 cm height) were installed within 50 cm of the NEE plots (Fig. 2). These were sealed to the peat surface in March 2013 using non-setting putty (Evo Stick “Plumbers Mait”, Stafford, UK). All collars (n = 24) had above-ground vegetation removed by regular clipping so they measured below-ground fluxes only. In addition, 56 cm diameter, 20 cm deep trenches were cut around half the collars to exclude live roots enabling measurement of the below-ground heterotrophic component. At each of six plots, the two replicates of each treatment were averaged to produce a single value for total soil (clipped) and heterotrophic soil (trenched and clipped) respiration. Repeated trenching was used to prevent root re-growth, rather than a barrier, to minimise the effect on the hydraulic properties of the peat.
Trenching and clipping were chosen as inexpensive, simple and established methods subject to well documented uncertainties (Kuzyakov and Larionova 2005; Subke et al. 2006) such as severing roots which decompose leading to an overestimation of heterotrophic respiration. Collars were installed 2 months prior to the start of sampling to reduce disturbance effects.
Soil CO2 flux measurements were taken on 17 separate dates in a randomised pattern approximately every 2 weeks from 13/05/2013 to 1/11/2013 and 14/04/2014 to 11/09/2014. CO2 flux was measured over 2 min using an EGM-4 infra-red gas analyser and a CPY-4 canopy assimilation chamber (PP Systems, Hitchin, UK). The autotrophic component of soil respiration was calculated from the difference between total and heterotrophic soil respiration measured at each location for each sample round.
Auxiliary measurements
Concurrently with both NEE and soil CO2 flux measurements soil temperature was recorded down a single vertical profile at 5, 10, 15, 20, 25 and 30 cm (Electronic Temperature Instruments, Worthing) below the peat surface in the vegetated haggs. In both the peat pans and the vegetated haggs the water table depth below the peat surface was measured using a ruler in a perforated tube. Water table depths were measured at nearby dipwells automatically every 15 min with in situ submersible water pressure transducers (IMSL Geo100 Impress, UK). Soil temperature was continuously measured at a depth of 15 cm every 15 min (Gemini Data Loggers, Chichester, UK) at vegetated plot 4. Rainfall was measured using a tipping bucket rain gauge (0.2 mm tip, RT1, Adcon Telemetry, Austria). Global irradiation was measured every 15 min (Adcon, Klosterneuburg, Austria). Data gaps were filled in by correlation (r2 = 0.91, p < 0.001) to the closest meteorological station, 18 km to the northwest and 340 m lower in elevation than the study site; North Wyke (UK Meteorological Office 2012) (50°46′N 3°54′W).
Vegetation composition, seasonal development and productivity
Vegetation composition and productivity
Visual inspection of the area inside the NEE collars in August 2013 assessed the percentage coverage of each species as well as total cover of bare ground, standing water, herbs (forbs), grasses, sedges, non-Sphagnum moss and Sphagnum moss. The number of species present at each location was counted to derive the species richness. The Shannon Diversity Index (Shannon 1948) (Eq. 2) and Inverse Simpson Diversity Index (Simpson 1949) (Eq. 3) were calculated; the first quantifies the uncertainty in predicting the next species, whilst the second describes the richness of a community increasing from 1, a community containing only one species.
where n is the number of species encountered and Pi the fraction of the entire population made up of species I, Ni is the total area of species i present and N the total area of vegetation.
Ellenberg’s Moisture Indicator Values (Hill et al. 1999) were determined for each location. Vascular species have been classified according to their ecological niche on a 12 point scale ranging from 1 (extreme dryness) to 12 (submerged plants). The classification values for the vascular species identified in this study were looked up and the average value for the species present at each location was calculated.
Destructive samples were collected to measure annual net primary productivity (ANPP) on 29/08/2013 and 07/08/2014. The timing was selected to coincide with flowering and peak biomass of the dominant vegetation, Molinia caerulea. All green material in a 0.2 × 0.2 m area near the CO2 collars (different location each year) was collected and oven dried at 78 °C to constant mass.
Vegetation seasonal development
Proxies for vegetation seasonal development were derived from Moderate Resolution Imaging Spectoradiometer (MODIS). MOD15A2 fPAR (1000 × 1000 m resolution) and MODIS9A1 surface reflectance (500 × 500 m resolution) were downloaded from USGS Earth Explorer (http://earthexplorer.usgs.gov). The normalised difference vegetation index was derived from bands 1 (Red) and 2 (near infra-red) of the surface reflectance where NDVI = (Band 2 − Band 1)/(band 2 + Band 1).
Data were screened and poor-quality data (cloudy, high aerosol concentrations or poor geometry) given a weighting of 0 and all other data a weighting of 1. To minimise variation due to atmospheric conditions, illumination and observation geometry a third order Fourier smoothing filter was applied. Points outside the 99% confidence interval were excluded. All remaining points (30 in 2013 and 23 in 2014) were then weighted equally and a Fourier third order series fitted to form a continuous daily timeseries (Gatis et al. 2017).
Seasonal net CO2 ecosystem exchange estimation
Seasonal NEE was modelled directly rather than modelling photosynthesis and ecosystem respiration separately and then combining them. Modelling the components separately requires the derivation of gross photosynthesis for each measurement based on the assumption that the full dark measurement represents ecosystem respiration. This adds additional uncertainty. As the closed chamber method measures NEE it was decided to work with these data directly. Given the binary nature of the system, NEE was parameterised for peat pans (n = 398) and vegetated haggs (n = 423) separately, using all the quality controlled closed chamber measurements collected at a range of light levels.
Linear, exponential, Arrhenius, Lloyd–Taylor and tolerance relationships were tested using combinations of NDVI, soil temperature at a range of depths and water table depths. The models selected had the greatest coefficient of regression, smallest root mean square errors and were the most parsimonious.
The NEE model for the vegetated haggs (Eq. 4) had two components, the first is dependent on NDVI and the second shows an Arrhenius relationship with soil temperature at 15 cm, the soil depth with the greatest correlation with ecosystem respiration).
The NEE model for the peat pan (Eq. 5) is also made up of two components, the first is dependent on NDVI; the second is dependent on soil temperature at 15 cm depth and water table depth.
where NEE is the net CO2 ecosystem exchange (μmol m−2 s−1), Pmax is the rate of light saturated photosynthesis (μmol m−2s−1), NDVI the normalised difference vegetation index, PPFD the incident PPFD (μmol Photons m−2 s−1), k the half-saturation constant of photosynthesis (μmol photons m−2 s−1), T15 soil temperature at a depth of 15 cm, a, b, c, d and f (dimensionless) are coefficients.
An hourly timeseries of PPFD was created by correlating episodic measurements taken concurrently with the flux measurements to global irradiation measured onsite (see Auxiliary measurements). Water table depths and soil temperature measured every 15 min (see Auxiliary measurements) were averaged to produce hourly time series. The growing season was defined as from the first three consecutive days with daily mean soil temperature > 10 °C till the first three consecutive days with daily soil temperature < 10 °C as this is the temperature at which root initiation and subsequent leaf growth occurs (Taylor et al. 2001).
Seasonal estimates were determined by the accumulation of hourly fluxes over the duration of the growing season. Model confidence intervals (95%) were determined from the root mean squared error of modelled values compared to measured values. Input parameter uncertainty was accounted for by using lower and upper 95 percentile values in the model. These two sources of uncertainty were summed together for each hour over the duration of the growing season. By convention CO2 fluxes are reported relative to the atmospheric pool so the peatland is a net CO2 source if positive.
Statistical analysis
To assess temporal and spatial variation in water table depth and soil temperature, a two-way repeated measures ANOVA was carried out with time as the within subject factor and landscape component (hagg or peat pan) as the between subject factor. To investigate potential temporal controls on CO2 fluxes, stepwise linear regressions were carried out on photosynthesis at a PPFD of 1000 μmol photons m−2 s−1 (PG1000), ecosystem respiration (REco) and total, heterotrophic and autotrophic below–ground respiration as measured and natural log-transformed. The temporal variables tested were water table depth, soil temperature at 5, 10, 15, 20, 25 and 30 cm, fPAR, NDVI, total PPFD in the preceding day and hour, total rainfall on the day of measurement (Rain0) and preceding 1, 7, 14 and 28 days. Exponential, Arrhenius and Lloyd–Taylor relationships between below-ground respiration and soil temperature at 5, 10, 15 20, 25 and 30 cm were also tested.
Results
Water table depth
Water tables were on average deeper and less variable in the vegetated haggs; mean of 9.1 ± 5.4 cm and varying from 28 cm below to 1 cm above ground level compared to a mean of 7.1 ± 10.1 cm and varying between 23 cm below to 21 cm above ground level in the peat pans (Fig. 3). During the 2013 growing season water table depths fell to a maximum low in July then rose again until late October 2013. Water table depths were generally higher in 2014 reaching a maximum in early June and a minimum in early July. Water tables dropped below the ground surface at all plots within the peat pans in July 2013. However, plots 5 and 6, which were located at a lower elevation than the other plots, had greater standing water depth and were more often saturated than the other bare peat plots. Water table depth varied significantly with time (two-way ANOVA p < 0.001) and between haggs and pans (p = 0.004).
Water table depth (cm below ground level) in the peat pans (top) and vegetated haggs (bottom) over the 2013 and 2014 growing seasons the six replicate plots within the study site (Fig. 1d)
Vegetation composition
Molinia caerulea was present in all vegetated NEE collars (8 to 50% coverage) and was the dominant vegetation in 5 out of 6 collars covering 20–50%. In vegetated collar 6 Narthecium ossifragum was the most common species (50%). Erica tetralix was the only other species present in all collars (3 to 20% coverage). In the peat pans either Eriophorum angustifolim and/or Sphagnum denticulatum was present. A full species list is provided in the supplementary material (Table 1). Shannon Diversity index, Inverse Simpson diversity index, and species richness were all greater in the vegetated haggs (Fig. 4a–c). This is due to the presence of herbs, grasses and mosses in the vegetated haggs but not in the peat pans (Fig. 4f, g and i). Although there was some vegetation present in all collars, bare ground and standing water were present in the peat pans but not the vegetated haggs (Fig. 4k and l). Ellenberg’s Moisture indicator values denoted the vegetated haggs were damp and the peat pans wet and often water saturated as observed. In the vegetated haggs above-ground annual net primary productivity (ANPP) was not significantly different between 2013 (217 ± 39 g m−2) and 2014 (214 ± 23 g m−2). ANPP was not assessed for the peat pans.
Seasonal CO2 fluxes
Photosynthesis at a PPFD equivalent to 1000 μmol Photons m−2 s−1 (PG1000) and ecosystem respiration (REco) followed similar seasonal patterns (Fig. 5) in the vegetated haggs, with REco and photosynthetic CO2 uptake (PG1000) increasing through late spring into summer. The greatest REco was recorded in July 2014 (3.6 ± 0.9 μmol m−2 s–1) whilst the greatest photosynthetic CO2 uptake was measured in September 2013 (− 6.1 ± 2.2 μmol m−2 s–1). Photosynthetic CO2 uptake peaked towards the end of the growing season (August and September) whilst REco peaked in mid-summer (July) reflecting seasonal temperature variation.
In the peat pans REco and photosynthetic CO2 uptake (PG1000) were lower than in the vegetated haggs (Fig. 5) except in July 2013 when there was a notable increase coinciding with low water tables (Fig. 3). Neither PG1000 nor REco showed a clear seasonal pattern.
Total and heterotrophic below-ground respiration in the vegetated haggs showed similar seasonal patterns generally rising from mid-May to late-August then decreasing to late-October in 2013 and rising from mid-April to mid-August in 2014 (Fig. 6). There was noticeably greater respiration in July 2013 when the soil temperature was greatest (17.6 °C), corresponding to the spike in photosynthetic CO2 uptake and ecosystem respiration observed in the peat pans (Fig. 5c). Heterotrophic respiration was greater than autotrophic respiration except during October 2013 and July and August 2014. The proportional contribution of autotrophic respiration to total soil respiration varied between 1 and 66% with the lowest contributions occurring in May 2013 and April 2014 and greater contributions later in the growing season. During the growing season autotrophic contributed on average 42%.
Temporal controls on CO2 fluxes
Ecosystem respiration and photosynthesis showed no significant relationships with water table depth in the vegetated haggs (p > 0.57) however, in the peat pans ecosystem respiration and photosynthetic CO2 uptake significantly increased when the water table fell (Table 1). This relationship was strongly driven by the high CO2 fluxes (Fig. 5) and deep water tables in July 2013 (Fig. 3). In the vegetated haggs photosynthetic CO2 uptake showed the strongest relationship with NDVI (Table 1), increasing when NDVI increased. Ecosystem respiration showed a significant exponential relationship with soil temperature at a depth of 15 cm (Table 1). Additional variables did not increase the coefficient of regression for photosynthesis or ecosystem respiration in the vegetated haggs or peat pans.
Of the soil temperature depths measured, total and heterotrophic below-ground respiration showed the strongest regression coefficients with an exponential function dependent on soil temperature at a depth of 5 cm (Fig. 7a and b) with respiration increasing as temperature increased. Autotrophic respiration, although significantly related to soil temperature at 5 cm (Fig. 7c), showed the strongest exponential relationship with soil temperature at 30 cm (Table 1). Multiple regression analysis indicated that water table depth was a stronger factor than soil temperature in controlling heterotrophic respiration (Table 1, Fig. 7e). Adding total rainfall on the preceding day increased the proportion of variability explained by 8%. Total and autotrophic below-ground respiration were also significantly related to water table depth (Fig. 7a and e) with higher respiration rates during dry conditions but their relationships with soil temperature were dominant (Table 1).
Seasonal net CO2 ecosystem exchange estimation
The model for the vegetated haggs (Eq. 4, Table 2), based on all the CO2 flux measurements collected explained a greater proportion of the variability (76%) than the model (Eq. 5, Table 2) for the peat pans (67%) however, it also had greater root mean square errors (Table 2). It can be seen that the model errors (Table 2) are large when compared to PG1000 and REco (Fig. 5) resulting in great uncertainty in the seasonal estimates (Table 3). It is estimated that it is most likely both the vegetated haggs and the peat pans were net CO2 sources over the 2013 and 2014 growing seasons (Table 3).
Discussion
Haggs and Pans: a binary system
Water table depths and vegetation composition
Average water table depth in the vegetated haggs (9.1 ± 0.4 cm) was shallower than those reported for inter-gully areas (23.4 ± 8 cm) (McNamara et al. 2008) and upslope of drainage ditches (19.8 ± 0.38 cm) (Coulson et al. 1990) in Calluna vulgaris dominated British blanket bogs. In addition, peat pans were frequently inundated (Fig. 3) suggesting these peat pans function differently to both gullies (McNamara et al. 2008; Dixon et al. 2013) and drainage ditches (Cooper et al. 2014) where the water table is more commonly below ground level except during storm events. It is likely the shallow topographic gradients and poor connectivity between peat pans (Fig. 1c) resulted in less water table drawdown in the peat pans when compared to both erosional gullies and drainage ditches (Parry et al. 2014). Despite this, hydrological monitoring at this site has shown that in the vegetated haggs the water table drops lower adjacent to the peat pans than further away (Luscombe, pers. comm. 2018). Sphagnum cover was < 20% in the vegetated haggs whilst cover of grasses and herbs reached 53 and 50% respectively (Fig. 4f, g and j) further indicating the deterioration of ecohydrological function in the vegetated haggs.
Vegetation in the peat pans was sparse (Fig. 4). This could be because active erosion (Foulds and Warburton 2007) removed peat preventing a continuous vegetation cover from developing (Ingram 1967). In addition, intermittent dry conditions (Fig. 3) may have made re-colonisation impossible for Sphagnum species (Price and Whitehead 2001). Eriophorum vaginatum has been shown to recolonise gullies starting from zones of redeposited peat (Crowe et al. 2008) and facilitate recolonization by other species (Tuittila et al. 2000). On Dartmoor, in nearby areas where lower connectivity has limited erosion and maintained more stable water tables, dense areas of Eriophorum angustifolim have formed. This suggests that if erosion could be halted and water tables stabilised then vegetation would be expected to recolonise these sparsely vegetated areas.
CO2 fluxes
Photosynthetic CO2 uptake and ecosystem respiration were lower in the peat pans than in the vegetated haggs (Fig. 5) even allowing for a 0.2 μmol m−2 s−1 uncertainty due to different CO2 chambers (15.7 l compared to 2.4 l) and analysers (Li-8100 compared to EGM-4). This difference was most likely driven by significant variation in vegetation diversity, species richness and vegetation cover (Fig. 4) due to different water table depths (Fig. 3).
Summer mean PG1000 (− 1.0 μmol m−2 s−1) from the peat pans was similar to an Eriophorum spp., Vaccinium myrtillus and bare peat naturally revegetating gully (− 1.1 to − 1.6 μmol m−2 s−1) (Clay et al. 2012; Dixon et al. 2015) but photosynthetic CO2 uptake was less than a rewet cut-away Eriophorum spp. dominated peatland (− 2.3 μmol m−2 s−1) (Wilson et al. 2016) and much less than a rewet cut-away Eriophorum spp. tussock (− 15.5 μmol m−2 s−1) (Tuittila et al. 1999). Given these annual results include large periods with PPFD levels below saturation it can be seen that these peat pans have low primary productivity even when compared to other damaged peatlands.
There was a notable spike in REco in late July 2013 (2.1 μmol m−2 s−1) (Fig. 5) coincident with warmer and drier conditions (Fig. 3). Although notably higher than other values recorded in this study, it is approximately half that reported for Eriophorum vaginatum in a naturally revegetated erosional gully (4.1 μmol m−2 s−1) (McNamara et al. 2008) under similar water table and temperature conditions. Growing season mean ecosystem respiration from the peat pans (0.5 μmol m−2 s−1) was greater than annual (0.2 to 0.4 μmol m−2 s−1) (Clay et al. 2012; Wilson et al. 2013; Dixon et al. 2015) and summer (0.04 μmol m−2 s−1) (Tuittila et al. 1999) REco rates for bare peat most likely due to some, albeit sparse, vegetation cover. However, compared to annual mean REco for an Eriophorum spp. and bare peat channel floor (0.6 μmol m−2 s−1) (Clay et al. 2012) and Eriophorum spp. and Sphagnum spp. rewet cut-away peat (0.5 m−2 s−1) (Wilson et al. 2016) the summer mean REco from the peat pans seems low. Again this probably reflects variation in vegetation cover and low primary productivity rather than differences in water tables directly, as the rewet peatland was wetter (− 9.5 to − 15.5 cm) (Wilson et al. 2016) than this study and the natural channel (13.9 cm) (Clay et al. 2012) drier.
Maximum REco in the vegetated haggs (3.6 μmol m−2 s−1) was similar to August REco from Moor House, a Calluna vulgaris, Eriophorum vaginatum and Sphagnum spp. upland blanket bog, (3.3 to 3.4 μmol m−2 s−1) (Hardie et al. 2009; Lloyd 2010). Summer mean REco (2.4 μmol m−2 s−1) was smaller than for a Vaccinium spp., Eriophorum vaginatum, Molinia caerulea and Calluna vulgaris upland bog (3.1 μmol m−2 s−1) (Urbanová et al. 2012) however, the mean water table was deeper in this drained bog (19.5 cm).
Heterotrophic respiration rates (Fig. 6) were generally lower on Dartmoor (mean and maximum of 0.8 and 2.7 μmol m−2 s−1) compared to those from August and September at Moor House (1.0 to 1.7 μmol m−2 s−1 (Hardie et al. 2009; Heinemeyer et al. 2011). This is surprising given the difference in water table depths; 0 to 8 cm at Moor House (Hardie et al. 2009) compared to − 1 to 28 cm in this study. It is possible variation in leaf litter quality (Ward et al. 2010) and quantity affected heterotrophic respiration rates. However, as the two studies at Moor House were based on only four sample events there is insufficient data to fully understand these differences.
Clipping and trenching severs roots which decompose leading to an overestimation of heterotrophic respiration (Kuzyakov and Larionova 2005; Subke et al. 2006) and therefore an underestimation of autotrophic respiration. Collars were installed 2 months prior to the start of sampling to reduce disturbance effects and the data do not show a systematic decrease in the proportion of heterotrophic respiration over time (Fig. 6) suggesting the effects were minimal.
Summer mean PG1000 (3.8 μmol m−2 s−1) in the vegetated haggs was similar to summer mean photosynthesis for a Vaccinium spp., Eriophorum vaginatum, Molinia caerulea and Calluna vulgaris upland bog (− 4.2 μmol m−2 s−1) (Urbanová et al. 2012), however this includes periods with lower PPFD so it is difficult to compare these values. Maximum photosynthetic CO2 uptake (PG1000) (− 6.1 μmol m−2 s−1) in this study was greater than maximum potential photosynthesis (Pmax) from Calluna vulgaris, Erica tetralix, Molinia caerulea and Sphagnum spp. hummocks in Irish blanket bog (− 4.2 μmol m−2 s−1) (Laine et al. 2006) but less than those reported for a Calluna vulgaris, Eriophorum vaginatum and Sphagnum spp. upland blanket bog (Moorhouse (− 16.3 to − 16.9 μmol m−2 s−1) (Lloyd 2010). The bogs in these studies had similar vegetation to this study (Molinia caerulea, Erica Tetralix, Eriophorum angustifolium, Calluna vulgaris) but given the sensitivity of photosynthesis to vegetation composition it is likely much of this variation is due to differences in the vegetation community present. However, above-ground annual net primary productivity was greater at Moor House, (approximately 300 g m−2) (Ward et al. 2007) than Dartmoor (214 ± 23 g m−2 in 2014) so the greater rates of photosynthesis may also in part be due to greater biomass, reflecting more optimum growing conditions.
The summer maximum photosynthetic CO2 uptake (PG1000) and REco measured in this study (Fig. 5a and b) were lower than those found on Exmoor (− 23.1 and 10.9 μmol m−2 s−1 respectively) (Gatis 2015), an upland also located within the south west of England. On Exmoor Molinia caerulea is more dominant and grows taller (up to 60 cm) than on Dartmoor (up to 20 cm). This is reflected in greater above-ground annual net primary productivity (ANPP); 517 ± 30 g m−2 on Exmoor. A greater quantity of leaf litter resulting from greater ANPP may also explain the higher rates of heterotrophic respiration on Exmoor (1.5 ± 0.1 μmol m−2 s−1) (Gatis 2015) where peat thickness is shallower (< 0.56 m). As high rates of photosynthesis have been found to increase autotrophic respiration (Subke et al. 2006) the difference in photosynthetic rates observed between these moors may explain the lower average autotrophic respiration rates from Dartmoor (Fig. 6) than Exmoor (1.3 ± 0.2 μmol m−2 s−1). No other values for autotrophic respiration could be found for comparison in this region, reflecting the large uncertainty in measuring autotrophic respiration (Subke et al. 2006).
Drivers of temporal variation in CO2 fluxes
In the vegetated haggs and peat pans, photosynthetic uptake at 1000 μmol Photons m−2 s−1 became significantly greater during periods of higher NDVI (Table 1). Photosynthesis has been related to vegetation seasonal development measured by NDVI in northern peatlands (up to 71% of variation explained) (Kross et al. 2013), NDVI in alpine grasslands (71% of variation explained) (Rossini et al. 2012), leaf area (Nieveen et al. 1998; Street et al. 2007; Otieno et al. 2009), vegetative green area (Riutta et al. 2007; Urbanová et al. 2012) and leaf biomass (Bubier et al. 2003). In the peat pans PG1000 showed no significant relationship with NDVI most likely due to the minimal vegetation cover.
In the peat pans, both ecosystem respiration and PG1000 (Table 1) showed the strongest relationships with water table depth. Photosynthetic CO2 uptake increased in the peat pans during dry periods (Table 1). Although Eriophorum angustifolium is a wetland species, evolved to live in waterlogged conditions, vegetation often close stomata in response to raised water tables, limiting gases exchange through the leaf surface (Pezeshki 2001). Photosynthetic uptake from Eriophorum vaginatum plots has been found to increase as water levels fall from 16.8 cm to a maximum at 14.6 cm below ground surface (Riutta et al. 2007). Where Eriophorum spp. plots were submerged following re-wetting of a cut-over peatland, vegetation cover initially decreased before increasing in the second year following re-wetting (Tuittila et al. 1999) suggesting Eriophorum spp. can adapt to submerged conditions but not instantaneously.
Water table depth has commonly been found to influence ecosystem respiration (Tuittila et al. 1999; Laine et al. 2006, 2007; Riutta et al. 2007; Wilson et al. 2007, 2013; Soini et al. 2010) with greater respiration occurring during drier conditions. Lowering the water table increases the depth to which oxygen can diffuse, thus enabling more rapid aerobic heterotrophic respiration to occur (Clymo 1983; Moore and Dalva 1993). In addition, greater rates of photosynthetic CO2 uptake and consequently autotrophic respiration also occurred during periods with lower water tables.
Total, heterotrophic and autotrophic below-ground respiration all showed significant exponential increase with soil temperature at 5 cm (Fig. 7a–c). Soil temperature measurements from shallower depths have been shown to be better predictors of respiration (Lafleur et al. 2005; Lloyd 2010) especially when the proportion of autotrophic respiration is greater, although autotrophic respiration showed the strongest exponential relationship with soil temperature at 30 cm; the deepest depth measured in this study (Table 1). Perhaps reflecting a mix of autotrophic and heterotrophic sources, ecosystem respiration showed the strongest exponential relationship with soil temperature at 15 cm. This is similar to the depth found by Updegraff et al. (2001) but deeper than other studies which found air temperature (Schneider et al. 2012); an average of air temperature and soil temperature at 20 cm (Laine et al. 2006); soil temperature at 5 cm (Bubier et al. 2003; Lund et al. 2007; Wilson et al. 2007) and 10 cm (Blodau et al. 2007; Otieno et al. 2009; Lloyd 2010) to have the strongest relationships.
Soil temperature and water table depths commonly co-vary and interact to amplify effects on below-ground respiration. For example, warm and dry conditions are often concurrent, with both conditions increasing rates of below-ground respiration (Fig. 7). Water table depths did show a significant relationship with total, heterotrophic and autotrophic soil respiration (Fig. 7) indicating respiration increased during periods of lower water tables. However, multiple regressions indicated that soil temperature was the primary control on below-ground autotrophic and total soil respiration suggesting the apparent relationship with water table depth may have been due to co-variation of water table depths and soil temperature.
Water table depth was the strongest control on heterotrophic respiration possibly due to increased aerobic heterotrophic respiration (Clymo 1983; Moore and Dalva 1993) and enhanced gas diffusion through oxygenated peat (Blodau and Moore 2003). Although below-ground respiration varied with water table, ecosystem respiration did not (Table 1) possibly due to different drivers affecting the multiple respiration sources that contribute to ecosystem respiration. This is consistent with other studies that have found temperature to be the main control on ecosystem respiration under wet conditions (Updegraff et al. 2001; Bubier et al. 2003) and within Molinia caerulea dominated systems (Nieveen et al. 1998) but in contrast to studies that found water level to have the strongest control over respiration (Silvola et al. 1996) or a small but significant effect (Lafleur et al. 2005; Otieno et al. 2009).
Seasonal net CO2 ecosystem exchange
Given the sparse vegetation cover (Fig. 4) it was unsurprising that the peat pans were gaseous CO2 (Table 3) as well as aquatic carbon sources (Malone, pers. comm. 2018) over the growing season. NEE fluxes (0.1 gCO2 m−2 d−1) were lower than those observed for bare Canadian cut-over peat (0.6 to 2.1 gCO2 m−2 d−1) (Waddington et al. 2010) and Eriophorum spp. tussock and inter-tussock plots in a Finnish cut-over peatland (0.3 to 1.2 gCO2 m−2 d−1) (Tuittila et al. 1999). This might be due to the limited vegetation cover as a closed Eriophorum spp. cover has been found to be a smaller net CO2 source (or even a net CO2 sink) compared to a non-vegetated surface under the same environmental conditions (Tuittila et al. 1999).
It was unexpected that the vegetated plots were a greater net CO2 source over the growing season (Table 3) given the greater vegetation cover (Fig. 4). However, Hardie et al. (2009) found 37–35% of summer ecosystem respiration flux to be from soil (RBG-ToT) suggesting 63–66% was from the vegetation so although the vegetated haggs had greater photosynthesis much of this would be rapidly re-released. In addition, root exudates add fresh organic matter to the subsurface stimulating microbes to decompose more recalcitrant peat (Fontaine et al. 2007). This would be enhanced by deeper water table depths in the vegetated haggs (Fig. 3) allowing oxygen to penetrate deeper into the peat resulting in increased decomposition (Silvola et al. 1996).
Other studies in a range of peatlands have also reported vegetated plots to be growing season net CO2 sources (Tuittila et al. 1999; Waddington et al. 2010; Urbanová et al. 2012; Strack and Zuback 2013). During measurements all sites were net CO2 sinks, however, these were collected during bright, daytime conditions. It should be noted that this model assumes the dependency of ecosystem respiration on temperature is the same in the day and night. Daily variation in autotrophic respiration (and primed heterotrophic respiration) has been shown to result in significant differences between day and night REco at the same temperatures (Juszczak et al. 2012; Wohlfahrt and Galvagno 2017). Therefore, it is most likely ecosystem respiration is overestimated by this model. In addition, shade cloths underestimate photosynthesis at low light levels compared to naturally low light conditions and a lack of temperature control within the chamber may have resulted in plant stress also underestimating photosynthesis. Consequently, this experimental design is biased towards overestimating CO2 release.
The models explained 76% of the variability in observed NEE in the vegetated haggs and 67% in the peat pans (Table 2) however, the root mean square errors are large in comparison to PG1000 and REco fluxes observed (Fig. 5). This has resulted in uncertainties many times larger than seasonal NEE estimates (Table 3). This uncertainty consists of both natural variability which is known to be significant when using multiple plots (Laine et al. 2009) and uncertainty associated with modelling. It has been shown that different treatment of closed chamber data can result in variation in estimated NEE of 0.25 gCO2 m−2 d−1 over annual estimates (Huth et al. 2017) sufficient to change the estimate of ecosystem exchange from a net CO2 source to a net CO2 sink. Accepting this uncertainty, the models suggest both landscape components are losing carbon with greater loss from the haggs even with a greater vegetation cover.
Given, that in this study, NEE was modelled for the growing season only, when the majority of carbon uptake occurs, Dartmoor would be expected to be a larger source over the whole year. This suggests peat pan formation and expansion has altered the ecohydrological functioning of the whole mire not just the eroded pan areas, altering the balance of CO2 uptake and release towards carbon loss. Ecohydrological restoration is required to prevent further carbon loss and promote a return to carbon sequestration.
In the UK restoration schemes have blocked erosional gullies using a combination of materials (peat, wood, stone, plastic piling and heather bales) to slow water flow, trap sediment and raise local water tables (Parry et al. 2014). This would be expected to halt the expansion of the peat pans and encourage peat deposition behind dams which should provide zones for colonisation by pioneering species such as Eriophorum spp. (Crowe et al. 2008) which may facilitate recolonization by other species (Tuittila et al. 2000). In rewet cut-away peatlands high and stable water tables have been found to rapidly increase Eriophorum spp. cover but also shift Eriophorum spp. dominated plots towards growing season net CO2 sinks (Tuittila et al. 1999; Waddington et al. 2010). In the vegetated haggs the response would be expected to vary with vegetation type (Komulainen et al. 1999) with raised water tables reducing respiration but also possibly photosynthesis. It should be noted that this study has focused on CO2, raising water tables has been shown to increase the release of CH4 particularly in areas of open water (Best and Jacobs 1997; Komulainen et al. 1998; Strack and Zuback 2013; Cooper et al. 2014; Wilson et al. 2016). However, in the longer-term, higher and more stable water tables might alter the vegetation present towards those associated with wetter conditions (Bellamy et al. 2012) and carbon sequestration.
Conclusion
This study aimed to investigate the spatial and temporal controls on CO2 fluxes in a climatically marginal, eroding blanket bog and to quantify CO2 fluxes from these landscape components. Understanding the effects of existing damage and the potential effects of restoration should enable more informed management choices to be made.
The water table was significantly higher in the peat pans than in the vegetated haggs resulting in clear differences in vegetation composition and productivity which lead to significant differences in photosynthesis and ecosystem respiration between these landscape components. CO2 fluxes in the peat pans were dominated by changes in water table depths whilst photosynthesis in the drier vegetated haggs was related to normalised difference vegetation index (a proxy for vegetation seasonal development). Although ecosystem respiration was strongly related to temperature, heterotrophic below-ground respiration significantly decreased as water tables rose suggesting higher, more stable water tables may reduce the peat being respired. An empirically derived net CO2 ecosystem exchange model suggests that over the growing seasons studied the drier vegetated haggs were a greater net CO2 source than the peat pans despite greater vegetation cover.
Peat pan formation and expansion has affected the ecohydrological functioning of the whole mire not just the eroded pan areas. This demonstrates the need to limit the spread of bare peat pans to protect the biodiversity of the mire, prevent further loss of stored carbon and promote a return to carbon sequestration.
Data Availability
The research data supporting this publication are openly available from the University of Exeter’s institutional repository at: https://doi.org/10.24378/exe.1143.
References
Bellamy PE, Stephen L, Maclean IS, Grant MC (2012) Response of blanket bog vegetation to drain-blocking. Appl Veg Sci 15:129–135. https://doi.org/10.1111/j.1654-109X.2011.01151
Best EPH, Jacobs FHH (1997) The influence of raised water table levels on carbon dioxide and methane production in ditch-dissected peat grasslands in the Netherlands. Ecol Eng 8:129–144. https://doi.org/10.1016/S0925-8574(97)00260-7
Blodau C, Moore TR (2003) Experimental response of peatland carbon dynamics to a water table fluctuation. Aquat Sci Res Across Boundaries 65:47–62. https://doi.org/10.1007/s000270300004
Blodau C, Roulet NT, Heitmann T et al (2007) Belowground carbon turnover in a temperate ombrotrophic bog. Glob Biogeochem Cycles 21:GB1021. https://doi.org/10.1029/2005gb002659
Bonnett SAF, Ross S, Linstead C, Maltby E (2009) A review of techniques for monitoring the success of peatland restoration. Technical Report, Natural England, SWIM-MER, Bristol
Bragg OM, Tallis JH (2001) The sensitivity of peat-covered upland landscapes. CATENA 42:345–360. https://doi.org/10.1016/S0341-8162(00)00146-6
Bubier JL, Bhatia G, Moore TR et al (2003) Spatial and temporal variability in growing-season net ecosystem carbon dioxide exchange at a large peatland in Ontario, Canada. Ecosystems 6:353–367. https://doi.org/10.1007/s10021-003-0125-0
Clark JM, Gallego-Sala AV, Allott TEH et al (2010) Assessing the vulnerability of blanket peat to climate change using an ensemble of statistical bioclimatic envelope models. Clim Res 45:131–U462. https://doi.org/10.3354/cr00929
Clay GD, Dixon S, Evans MG et al (2012) Carbon dioxide fluxes and DOC concentrations of eroding blanket peat gullies. Earth Surf Process Landforms 37:562–571. https://doi.org/10.1002/esp.3193
Clymo RS (1983) Peat. In: Gore AJP (ed) Mires, Swamp, Fen and Moor. General studies. Ecosystems of the World 4a. Elsevier Scientific, Amsterdam, pp 159–224
Cooper MDA, Evans CD, Zielinski P et al (2014) Infilled ditches are hotspots of landscape methane flux following peatland re-wetting. Ecosystems 17:1227–1241. https://doi.org/10.1007/s10021-014-9791-3
Coulson JC, Butterfield J (1978) An investigation of the biotic factors determining the rates of plant decomposition on blanket bog. J Ecol 66:631–650. https://doi.org/10.2307/2259155
Coulson JC, Butterfield JEL, Henderson E (1990) The effect of open drainage ditches on the plant and invertebrate communities of moorland and on the decomposition of peat. J Appl Ecol 27:549–561. https://doi.org/10.2307/2404301
Crowe SK, Evans MG, Allott TEH (2008) Geomorphological controls on the re-vegetation of erosion gullies in blanket peat: implications for bog restoration. Mires Peat 3:1–14
Daniels SM, Agnew CT, Allott TEH, Evans MG (2008) Water table variability and runoff generation in an eroded peatland, South Pennines, UK. J Hydrol 361:214–226. https://doi.org/10.1016/J.JHYDROL.2008.07.042
Dixon SD, Qassim SM, Rowson JG et al (2013) Restoration effects on water table depths and CO2 fluxes from climatically marginal blanket bog. Biogeochemistry 118:1–18. https://doi.org/10.1007/s10533-013-9915-4
Dixon SD, Worrall F, Rowson JG, Evans MG (2015) Calluna vulgaris canopy height and blanket peat CO2 flux: implications for management. Ecol Eng 75:497–505. https://doi.org/10.1016/j.ecoleng.2014.11.047
Evans MG, Burt TP, Holden J, Adamson JK (1999) Runoff generation and water table fluctuations in blanket peat: evidence from UK data spanning the dry summer of 1995. J Hydrol 221:141–160. https://doi.org/10.1016/S0022-1694(99)00085-2
Evans MG, Warburton J, Yang J (2006) Eroding blanket peat catchments: global and local implications of upland organic sediment budgets. Geomorphology 79:45–57. https://doi.org/10.1016/J.GEOMORPH.2005.09.015
Fontaine SS, Barot SS, Barré P et al (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280. https://doi.org/10.1038/nature06275
Foulds SA, Warburton J (2007) Wind erosion of blanket peat during a short period of surface desiccation (North Pennines, Northern England). Earth Surf Process Landforms 32:481–488. https://doi.org/10.1002/esp.1422
Gatis N (2015) Determining the effects of peatland restoration on carbon dioxide exchange and potential for climate change mitigation. University of Exeter, Exeter
Gatis N, Anderson K, Grand-Clement E et al (2017) Evaluating MODIS vegetation products using digital images for quantifying local peatland CO2 gas fluxes. Remote Sens Ecol Conserv 3:217–231. https://doi.org/10.1002/rse2.45
Gatis N, Luscombe DJ, Carless D et al (2019) Mapping upland peat depth using airborne radiometric and LiDAR survey data. Geoderma 335:78–87
Grand-Clement E, Anderson K, Smith D et al (2013) Evaluating ecosystem goods and services after restoration of marginal upland peatlands in South-West England. J Appl Ecol. https://doi.org/10.1111/1365-2664.12039
Hardie SML, Garnett MH, Fallick AE et al (2009) Bomb-C14 analysis of ecosystem respiration reveals that peatland vegetation facilitates release of old carbon. Geoderma 153:393–401. https://doi.org/10.1016/j.geoderma.2009.09.002
Heinemeyer A, Di Bene C, Lloyd AR et al (2011) Soil respiration: implications of the plant-soil continuum and respiration chamber collar-insertion depth on measurement and modelling of soil CO2 efflux rates in three ecosystems. Eur J Soil Sci 62:82–94. https://doi.org/10.1111/j.1365-2389.2010.01331.x
Hill MO, Mountford JO, Roy DB, Bunce RGH (1999) Factors controlling biodiversity in the British countryside (ECOFACT), volume 2: Technical Annex—Ellenberg’s indicator values for British plants. Institute of Terrestrial Ecology, Huntingdon
Holden J, Shotbolt L, Bonn A et al (2007) Environmental change in moorland landscapes. Earth-Sci Rev 82:75–100. https://doi.org/10.1016/j.earscirev.2007.01.003
Holden J, Wallage ZE, Lane SN, McDonald AT (2011) Water table dynamics in undisturbed, drained and restored blanket peat. J Hydrol 402:103–114. https://doi.org/10.1016/j.jhydrol.2011.03.010
Huth V, Vaidya S, Hoffmann M et al (2017) Divergent NEE balances from manual-chamber CO2 fluxes linked to different measurement and gap-filling strategies: a source for uncertainty of estimated terrestrial C sources and sinks? Z Pflanzenernahr Bodenkd 180:302–315. https://doi.org/10.1002/jpln.201600493
Ingram HAP (1967) Problems of hydrology and plant distribution in mires. J Ecol 55:711–724
Juszczak R, Acosta M, Olejnik J (2012) Comparison of daytime and nighttime ecosystem respiration measured by the closed chamber technique on a temperate mire in Poland. Polish J Environ Stud 21:643–658
Komulainen V-M, Nykänen H, Martikainen PJ, Laine J (1998) Short-term effect of restoration on vegetation change and methane emissions from peatlands drained for forestry in southern Finland. Can J For Res 28:402–411
Komulainen VM, Tuittila ES, Vasander H, Laine J (1999) Restoration of drained peatlands in southern Finland: initial effects on vegetation change and CO2 balance. J Appl Ecol 36:634–648. https://doi.org/10.1046/j.1365-2664.1999.00430.x
Kross A, Seaquist JW, Roulet NT et al (2013) Estimating carbon dioxide exchange rates at contrasting northern peatlands using MODIS satellite data. Remote Sens Environ 137:234–243. https://doi.org/10.1016/j.rse.2013.06.014
Kuzyakov Y, Larionova AA (2005) Root and rhizomicrobial respiration: a review of approaches to estimate respiration by autotrophic and heterotrophic organisms in soil. J Plant Nutr Soil Sci 168:503–520. https://doi.org/10.1002/jpln.200421703
Lafleur PM, Moore TR, Roulet NT, Frolking S (2005) Ecosystem respiration in a cool temperate bog depends on peat temperature but not water table. Ecosystems 8:619–629. https://doi.org/10.1007/s10021-003-0131-2
Laine A, Sottocornola M, Kiely G et al (2006) Estimating net ecosystem exchange in a patterned ecosystem: example from blanket bog. Agric For Meteorol 138:231–243. https://doi.org/10.1016/j.agrformet.2006.05.005
Laine A, Byrne KA, Kiely G, Tuittila E-SS (2007) Patterns in vegetation and CO2 dynamics along a water level gradient in a lowland blanket bog. Ecosystems 10:890–905. https://doi.org/10.1007/s10021-007-9067-2
Laine A, Riutta T, Juutinen S et al (2009) Acknowledging the spatial heterogeneity in modelling/reconstructing carbon dioxide exchange in a northern aapa mire. Ecol Modell 220:2646–2655. https://doi.org/10.1016/j.ecolmodel.2009.06.047
Lindsay RA, Charman DJ, Everingham F et al (1988) The flow country; the peatlands of Caithness and Sutherland. Nat Conserv Counc Peterbrgh. https://doi.org/10.1016/0006-3207(89)90043-8
Lloyd AR (2010) Carbon fluxes at an upland blanket bog in the north Pennines. Durham University, Durham
Lund M, Lindroth A, Christensen TR et al (2007) Annual CO2 balance of a temperate bog. Tellus B 59:804–811. https://doi.org/10.1111/j.1600-0889.2007.00303.x
McNamara NP, Plant T, Oakley S et al (2008) Gully hotspot contribution to landscape methane (CH4) and carbon dioxide (CO2) fluxes in a northern peatland. Sci Total Environ 404:354–360. https://doi.org/10.1016/j.scitotenv.2008.03.015
Moore PD (1987) Ecological and hydrological aspects of peat formation. Geol Soc Lond 32:7–15. https://doi.org/10.1144/gsl.sp.1987.032.01.02
Moore TR, Dalva M (1993) The influence of temperature and water table position on carbon dioxide and methane emissions from laboratory columns of peatland soils. J Soil Sci 44:651–664. https://doi.org/10.1111/j.1365-2389.1993.tb02330.x
Nieveen JP, Jacobs CMJ, Jacobs AFG (1998) Diurnal and seasonal variation of carbon dioxide exchange from a former true raised bog. Glob Chang Biol 4:823–833. https://doi.org/10.1046/j.1365-2486.1998.00189.x
Otieno DO, Wartinger M, Nishiwaki A et al (2009) Responses of CO2 exchange and primary production of the ecosystem components to environmental changes in a mountain peatland. Ecosystems 12:590–603. https://doi.org/10.1007/s10021-009-9245-5
Parry LE, Charman DJ, Noades JPW (2012) A method for modelling peat depth in blanket peatlands. Soil Use Manag 28:614–624. https://doi.org/10.1111/j.1475-2743.2012.00447.x
Parry LE, Holden J, Chapman PJ (2014) Restoration of blanket peatlands. J Environ Manage 133:193–205. https://doi.org/10.1016/j.jenvman.2013.11.033
Pezeshki SR (2001) Wetland plant responses to soil flooding. Environ Exp Bot 46:299–312. https://doi.org/10.1016/S0098-8472(01)00107-1
Price JS, Whitehead GS (2001) Developing hydrologic thresholds for Sphagnum recolonization on an abandoned cutover bog. Wetlands 21:32–40. https://doi.org/10.1672/0277-5212(2001)021%5b0032:DHTFSR%5d2.0.CO;2
Riutta T, Laine J, Aurela M et al (2007) Spatial variation in plant community functions regulates carbon gas dynamics in a boreal fen ecosystem. Tellus Ser B Chem Phys Meteorol 59:838–852. https://doi.org/10.1111/j.1600-0889.2007.00302.x
Rodwell JS (1991) British plant communities: mires and heaths, 2nd edn. Cambridge University Press, Cambridge
Rossini M, Cogliati S, Meroni M et al (2012) Remote sensing-based estimation of gross primary production in a subalpine grassland. Biogeosciences 9:2565–2584. https://doi.org/10.5194/bg-9-2565-2012
Rowson JG, Worrall F, Evans MG (2013) Predicting soil respiration from peatlands. Sci Total Environ 442:397–404. https://doi.org/10.1016/j.scitotenv.2012.10.021
Schneider J, Kutzbach L, Wilmking M (2012) Carbon dioxide exchange fluxes of a boreal peatland over a complete growing season, Komi Republic, NW Russia. Biogeochemistry 111:485–513. https://doi.org/10.1007/s10533-011-9684-x
Schuur EAG, Bockheim J, Canadell JG et al (2008) Vulnerability of permafrost carbon to climate change: implications for the global carbon cycle. Bioscience 58:701–714
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423
Silvola J, Alm J, Ahlholm U et al (1996) CO2 fluxes from peat in boreal mires under varying temperature and moisture conditions. J Ecol 84:219–228. https://doi.org/10.2307/2261357
Simpson EH (1949) Measurement of diversity. Nature 163:988
Soini P, Riutta T, Yli-Petäys M et al (2010) Comparison of vegetation and CO2 dynamics between a restored cut-away peatland and a pristine fen: evaluation of the restoration success. Restor Ecol 18:894–903. https://doi.org/10.1111/j.1526-100X.2009.00520.x
Stevenson AC, Jones VJ, Battarbee RW (1990) The cause of peat erosion: a palaeolimnological approach. New Phytol 114:727–735
Strack M, Zuback YCA (2013) Annual carbon balance of a peatland 10 yr following restoration. Biogeosciences 10:2885–2896. https://doi.org/10.5194/bg-10-2885-2013
Street LE, Shaver GR, Williams M, Van Wijk MT (2007) What is the relationship between changes in canopy leaf area and changes in photosynthetic CO2 flux in arctic ecosystems? J Ecol 95:139–150. https://doi.org/10.1111/j.1365-2745.2006.01187.x
Subke J-AA, Inglima I, Cotrufo MF (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a metaanalytical review. Glob Chang Biol 12:921–943. https://doi.org/10.1111/j.1365-2486.2006.01117.x
Tallis JH (1997) The southern Pennine experience: an overview of blanket mire degradation. In: Tallis JH, Meade R, Hulme PD (eds) Blanket mire degradation-causes, consequences and challenges. Proceedings of a conference at University Of Manchester, 9–11 April, 1979. The Macaulay Land Use Research Institute on behalf of the Mires Research Group, Aberdeen, pp 7–15
Taylor K, Rowland AP, Jones HE (2001) Molinia caerulea (L.) Moench. J Ecol 89:126–144. https://doi.org/10.1046/j.1365-2745.2001.00534.x
Thormann MN, Szumigalski AR, Bayley SE (1999) Aboveground peat and carbon accumulation potentials along a bog-fen-marsh wetland gradient in southern boreal Alberta, Canada. Wetlands 19:305–317. https://doi.org/10.1007/BF03161761
Tuittila ES, Komulainen VM, Vasander H, Laine J (1999) Restored cut-away peatland as a sink for atmospheric CO2. Oecologia 120:563–574. https://doi.org/10.1007/s004420050891
Tuittila E, Rita H, Vasander H, Laine J (2000) Vegetation patterns around Eriophorum vaginatum L. tussocks in a cut-away peatland in southern Finland. Can J Bot 78:47–58. https://doi.org/10.1139/b99-159
UK Meteorological Office (2012) Meteorologial Office Integrated Data Archive System (MIDAS) land and marine surface stations data (1853-current). http://badc.nerc.ac.uk/view/badc.nerc.ac.uk__ATOM__dataent_ukmo-midas
Updegraff K, Bridgham SD, Pastor J et al (2001) Response of CO2 and CH4 emissions from peatlands to warming and water table manipulation. Ecol Appl 11:311–326. https://doi.org/10.2307/3060891
Urbanová Z, Picek T, Hájek T et al (2012) Vegetation and carbon gas dynamics under a changed hydrological regime in central European peatlands. Plant Ecol Divers 5:89–103. https://doi.org/10.1080/17550874.2012.688069
Waddington JM, Strack M, Greenwood MJ (2010) Toward restoring the net carbon sink function of degraded peatlands: short-term response in CO2 exchange to ecosystem-scale restoration. J Geophys Res Biogeosciences 115:G01008. https://doi.org/10.1029/2009jg001090
Wallen B (1993) Methods for studying below-ground production in mire ecosystems. Suo 43:155–162
Ward SE, Bardgett RD, McNamara NP et al (2007) Long-term consequences of grazing and burning on northern peatland carbon dynamics. Ecosystems 10:1069–1083. https://doi.org/10.1007/s10021-007-9080-5
Ward SE, Ostle NJ, McNamara NP, Bardgett RD (2010) Litter evenness influences short-term peatland decomposition processes. Oecologia 164:511–520. https://doi.org/10.1007/s00442-010-1636-y
Wilson D, Tuittila E-S, Alm J et al (2007) Carbon dioxide dynamics of a restored maritime peatland. Ecoscience 14:71–80. https://doi.org/10.2980/1195-6860(2007)14%5b71:CDDOAR%5d2.0.CO;2
Wilson D, Farrell C, Mueller C et al (2013) Rewetted industrial cutaway peatlands in western Ireland: a prime location for climate change mitigation? Mires Peat 11:1–22
Wilson D, Farrell CA, Fallon D et al (2016) Multiyear greenhouse gas balances at a rewetted temperate peatland. Glob Chang Biol 22:4080–4095. https://doi.org/10.1111/gcb.13325
Wohlfahrt G, Galvagno M (2017) Revisiting the choice of the driving temperature for eddy covariance CO2 flux partitioning. Agric For Meteorol 237–238:135–142. https://doi.org/10.1016/J.AGRFORMET.2017.02.012
Yeloff DE, Labadz JC, Hunt CO (2006) Causes of degradation and erosion of a blanket mire in the southern Pennines. UK, Mires Peat, p 1
Acknowledgements
The authors would like to thank the members of the Dartmoor Peatland Partnership for their help. This research received financial support from South West Water Ltd, The University of Exeter (SK05284 and SK04809) and the Knowledge Transfer Partnership programme (KTP 8099). This KTP was funded by the Technology Strategy Board and the Natural Environment Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gatis, N., Benaud, P., Ashe, J. et al. Assessing the impact of peat erosion on growing season CO2 fluxes by comparing erosional peat pans and surrounding vegetated haggs. Wetlands Ecol Manage 27, 187–205 (2019). https://doi.org/10.1007/s11273-019-09652-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-019-09652-9