Abstract
Microalgae represent an alternative to conventional wastewater treatment, potentially improving antibiotic removal and offering a solution to combat the spread of antimicrobial resistance. Through batch assays, this study investigates the routes for antibiotic removal using three strains (Chlamydomonas acidophila, Auxhenochlorella protothecoides and Tetradesmus obliquus). Using mixtures of ciprofloxacin, clarithromycin, erythromycin, metronidazole, ofloxacin, sulfamethoxazole, and trimethoprim at concentrations simulating wastewater composition, it also assesses antibiotic effects on microalgae physiology. The three strains primarily removed antibiotics through rapid biosorption, achieving up to 91.5% removal for specific ones like ciprofloxacin. T. obliquus and C. acidophila showed efficacy, with total removals of 37.2% and 49.3%, respectively. Over time, A. protothecoides demonstrated the highest active removal efficiency, eliminating 22.1% of total antibiotics, with a notable 67.6% removal for sulfamethoxazole. Abiotic degradation through hydrolysis and photolysis contributed to ciprofloxacin, ofloxacin, clarithromycin, and erythromycin removal (34.7% to 96.7%), showing pH-dependent photolysis. However, algae induced a shading effect, reducing the photolytic and hydrolytic degradation of specific antibiotics. T. obliquus and C. acidophila were inhibited by antibiotics, whereas A. protothecoides showed a 30.6% growth rate increase. The stimulatory effect was also observed for the nutrient removal, with A. protothecoides showing a 46.6% increase in ammonium removal and a 44.8% increase in phosphate removal with antibiotics. Additionally, antioxidant activities remained stable, except for a notable increase in peroxidase activity for A. protothecoides and T. obliquus. The study confirms efficient antibiotic removal and stimulatory responses in the three algal strains, indicating their potential for wastewater treatment and combating antimicrobial resistance.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Rapid population growth and increased human activities have caused a significant rise in water pollution in many countries, with wastewater (WW) as one of the primary sources (Qv et al., 2023). Every year, the world produces about 360 billion cubic meters of untreated WW, and nearly half of this volume is discharged into the environment without undergoing treatment (Jones et al., 2021). Due to the presence of carbon, nitrogen, and phosphorus, WW can trigger eutrophication and harm ecosystems if left untreated (Preisner et al., 2020). Additionally, it contains antibiotics recently identified as a hazard to humans and aquatic life (Tran et al., 2016). A recent systematic review identified seven high-risk antibiotics commonly found in WWTP influents and effluents, including ciprofloxacin, clarithromycin, erythromycin, metronidazole, ofloxacin, sulfamethoxazole, and trimethoprim, which pose significant risks due to their prevalence, high concentrations, elevated environmental detrimental impact, and resistance to removal treatments (Frascaroli et al., 2021). These antibiotics can trigger antimicrobial resistance (AMR), which poses a significant threat to human health (Larsson & Flach, 2022). Predictive models suggest around 4.95 million deaths associated with bacterial AMR worldwide in 2019, including 1.27 million directly attributed to bacterial AMR (Antimicrobial Resistance Collaborators, 2022). By the year 2050, this figure is anticipated to reach 10 million (O’Neill, 2016). In Europe, AMR results in severe illnesses, prolonged hospital admissions, elevated healthcare costs, increased expenses for second-line drugs, and treatment failures, amounting to an estimated annual cost exceeding 9 billion euros (Dadgostar, 2019). Conventional WW treatment plants (WWTPs) employing physical, chemical, and biological approaches are ineffective at removing antibiotics (Li et al., 2019; Tran et al., 2016). This underscores the urgent need for improved studies to mitigate the environmental and health risks associated with these contaminants while treating WW.
Microalgae-based technology has emerged as a promising solution to integrate with current WWTPs due to its capability to remove pollutants and nutrients, reducing levels of chemical and biological oxygen demand, efficient CO2 capture, eco-friendly nature, and potential for high-value products (Ji et al., 2020; Kotoula et al., 2020; Walls et al., 2019). In particular, microalgae-based techniques have gained recognition for their cost-effective simultaneous remediation of nutrients and antibiotics (Leng et al., 2020; Qv et al., 2023). With a growing emphasis on achieving net-zero objectives, the appeal of a WW treatment that can both treat WW and eliminate antibiotics concurrently is substantial. For example, species like Auxenochlorella protothecoides, though scarcely studied for antibiotic removal, have demonstrated pollutant and nutrient recovery from WW while concurrently accumulating CO2 in valuable biomass rich in lipids (Zhou et al., 2012). On the other hand, Tetradesmus obliquus is one of the most extensively investigated species for coupling antibiotic removal and WW treatment (Ali et al., 2018; Silva et al., 2020; Xiong et al., 2019). The mechanisms for antibiotic removal depend on the microalgae species and antibiotic properties, with different mechanisms impacting efficiency (Maryjoseph & Ketheesan, 2020). Notably, pH conditions in WW play a pivotal role, as they can be adjusted to support optimal microalgae growth, influencing the removal by altering antibiotic functional group protonation (Vassalle et al., 2020). Certain species, such as Chlamydomonas acidophila, thrive at lower pH levels, offering an opportunity to explore new pH conditions for WW treatment that could enhance antibiotic removal (Escudero et al., 2020). Existing research primarily focuses on removing antibiotics using well-known algae species. However, there is a significant gap in understanding the specific removal routes in comparative studies using species scarcely investigated for this purpose, compared with well-known species under different conditions. In addition, abiotic factors, depending on the conditions, crucially influence antibiotic removal (Yang et al., 2021); however, the distinction between biotic and abiotic factors in these mechanisms is often unclear. Exploring different strains and pH scenarios can deepen our understanding of antibiotic removal mechanisms, optimising the process to identify effective microalgae strains for potential implementation in WW treatment.
The presence of antibiotics in WW not only represents a problem for AMR development but can hinder the overall performance of algae in WW treatment, leading to suboptimal results, especially in terms of nutrient recovery and biomass productivity. For instance, Chlorella sp. exhibited inhibited growth in the presence of tetracycline, which negatively affected nutrient removal. (Wang et al., 2021). Furthermore, high levels of antibiotics can disrupt vital processes like photosynthesis, generating reactive oxygen species (ROS), leading to the collapse of the culture (Zhang et al., 2021). Microalgae counteract this overproducing pigments or utilising antioxidant enzymes like glutathione S-transferase (GST) and peroxidase (POX) to regulate ROS and protect cells (Aderemi et al., 2018). However, excessive ROS can damage lipids, proteins, and DNA, impacting microalgae growth and metabolism, with malondialdehyde (MDA) serving as a biomarker for oxidative stress resulting from lipid peroxidation (Ayala et al., 2014). These detrimental effects can significantly reduce microalgal growth, thereby impacting WW treatment efficiency in terms of pollutants and nutrient removal. Hence, it is crucial to assess how antibiotics affect the antioxidative responses of microalgae. Moreover, understanding the impact of antibiotic mixtures on microalgae at environmentally relevant concentrations is crucial for evaluating the feasibility of microalgae-based WW treatment solutions. Recent studies have demonstrated the dual role of aquatic pollutants on microalgae, where high doses can adversely affect growth, while low doses can stimulate beneficial reactions such as growth in these organisms (Mao et al., 2021; Xu et al., 2022). This dose–response mechanism, known as hormesis, occurs when stressors such as antibiotics are tolerated by organisms within specific ranges. At low levels, the stressors induce the production of protective compounds, such as pigments, or metabolic changes in algae, leading to enhanced growth (Kiki et al., 2022; Le et al., 2023; Frascaroli et al., 2024). However, when the concentration of these compounds exceeds tolerable levels, inhibition is observed (Manzi et al., 2022). While much of the previous research used antibiotic concentrations well above those found in WW, research is needed to understand how antibiotics at real WW concentrations affect microalgae-based WW treatment.
The primary goal of this research was to evaluate the efficacy of three algae strains (C. acidophila, A. protothecoides, and T. obliquus) known for WW treatment in eliminating seven targeted antibiotics commonly found in WW (ciprofloxacin, clarithromycin, erythromycin, metronidazole, ofloxacin, sulfamethoxazole, and trimethoprim). This was achieved by investigating antibiotic removal routes and assessing the impact of antibiotics on growth, nutrient removal and antioxidative responses. In essence, this study lays the foundation for the potential use of these strains in WW treatment, particularly in addressing antibiotic pollution and AMR.
2 Materials and Methods
2.1 Microalgae Species and Cultivation Conditions
The strains of Chlamydomonas acidophila (CCAP 11/133), Auxenochlorella protothecoides (CCAP 211/7A) and Tetradesmus obliquus (CCAP 276/6B) were procured from the Culture Collection of Algae and Protozoa (Oban, UK). Cultivation was carried out using sterile BG − 11 medium in 1L bottles equipped with a filtered air bubbling system to ensure continuous cell suspension. The bottles were illuminated using a fluorescent lamp at an intensity of 4000 lx, following a 16 h/8 h light/dark cycle, and the temperature was maintained at 21 ± 3 °C.
2.2 Chemicals and Reagents
Ciprofloxacin (CIP), clarithromycin (CLA), ofloxacin (OFL), and trimethoprim (TMP) were procured from Sigma-Aldrich (St. Louis, USA). Erythromycin (ERY) and metronidazole (MDZ) were sourced from Acros Organics (Geel, Belgium). Sulfamethoxazole (SMX) was obtained from MP Biomedicals (Santa Ana, USA). The antibiotics had a purity exceeding 98%, except for clarithromycin, which exceeded 95%. Optima-grade methanol, acetonitrile, and formic acid were procured from Fisher Scientific (Waltham, USA) for antibiotic analysis. The various chemicals for antioxidative factors nutrient removal analysis were supplied by Sigma-Aldrich (St. Louis, USA) and Fisher Scientific (Waltham, USA). Solutions of these reagents were optimally stored for a maximum of two weeks, and Ultrapure water, generated with an Elga Purelab system (High Wycombe, UK), was used to prepare all solutions and media.
2.3 Experimental Setup
Batch culture experiments were conducted to investigate the antibiotic removal and the response mechanisms, encompassing growth, nutrient removal and antioxidative activity in microalgae. Upon reaching the exponential growth phase, microalgal cells were harvested and separated from the mother culture through centrifugation. These cells were inoculated into 500 mL Erlenmeyer flasks at an initial concentration of 2.5 × 106 cells mL−1. The flasks were filled with 300 mL of sterilised modified BG − 11 medium. The nutrient concentration in this medium was modified to mimic typical municipal WW conditions: 60 mg L−1 of ammonium, 1.5 mg L−1 of nitrate and 5 mg L−1 of phosphorous (as detailed in Supplementary Information, Table S1) (Frascaroli et al., 2024; Huang et al., 2015). Then, the pH of the media was modified to two distinct levels based on the microalgae species employed. Given that C. acidophila is an acidophilic species (Negoro, 1944), two WW scenarios were simulated: one at pH 3.2 specifically for C. acidophila and another at pH 7 for A. protothecoides and T. obliquus. The selection of the acidic pH aimed to create the optimal condition for the acidophilic species, opening the possibility of investigating how the application of acidity could affect the WW treatment, particularly in antibiotic removal. Inversely, a neutral pH of 7.0 was maintained for the second scenario to explore potential variances in removal efficiency compared to the acidic scenario.
In alignment with prior research (Frascaroli et al., 2024), a concentration of 100 µg L−1 for each of the seven antibiotics was added to simulate a high-stress situation. Fresh antibiotic solutions at 1 mg L−1 were prepared at the beginning of the experiment using ultrapure water and 1% formic acid to facilitate the dissolution of antibiotics in the aqueous medium. The seven antibiotics were then combined to create a mixture at 1 mg L−1. Aliquots of this mixture were added to flasks containing modified BG − 11 medium with and without algae to achieve a final concentration of 100 µg L⁻1 for each antibiotic. Flasks containing algae without antibiotics were used as controls. Flasks with algae and antibiotics were used to test for biotic removal. Flasks with antibiotics but no algae were used to test abiotic antibiotic degradation. Abiotic hydrolysis was assessed in experiments using flasks covered with a black nitrile film, while photolysis was determined in flasks exposed to the same light conditions as those with algae. These experiments were carried out using sterilised modified BG − 11 medium at the two pH values of 3.2 and 7. The experimental setup is summarised in Table 1.
Utilising the methodology detailed in Supplementary Information (Supplemental Methods SM1) based on preceding research, abiotic degradation, including photolysis and hydrolysis, and biotic mechanisms such as biosorption and active removal were calculated (Frascaroli et al., 2024). Three replicas for each treatment were arranged. The flasks were incubated for 9 days in triplicate in an Incu-Shake FL24 − 1 shaking incubator from SciQuip (Newtown, UK), maintained at 120 rpm and 23 ± 3°C. The incubator provided constant illumination using natural light with white LED at 4000 lx.
All the parameters related to the growth, antibiotic and nutrient removal and antioxidative activity were analysed thrice on days 0, 3, 6 and 9.
2.4 Analytical Procedures
2.4.1 Determination of Antibiotic Concentrations
Samples (3 mL) for the assessment of antibiotic removal were acquired following spiking and homogenisation immediately after 10 min from spiking and at 3, 6, and 9 days after the start of the experiment. After collection, the samples underwent centrifugation at 2,000 rpm for 20 min. The resultant pellet was utilised to evaluate antioxidative activity, while the supernatant was employed to assess antibiotic and nutrient removal. For the analysis of antibiotic removal, 3 mL of supernatant underwent solid-phase extraction (SPE) using Oasis HLB 3 cc cartridges (60 mg sorbent, 30 µm) obtained from Waters (Milford, USA). Afterwards, the samples were reconstituted in a mixture of acetonitrile and water (10:90) and stored at − 20 °C for a maximum of two weeks until analysis. Detailed information regarding the SPE procedure, recovery rates, matrix effects, and acceptance criteria for the limit of quantification for each antibiotic can be found in the Supplementary Information (Fig S1, Tables S2-4). Liquid chromatography coupled with mass spectrometry (LC–MS) was employed to analyse antibiotic concentrations in the samples. This utilised a Thermo Scientific Q-Exactive Orbitrap mass (Waltham, USA). LC–MS conditions are described in the Supplementary Information (Table S5).
2.4.2 Extraction of Antioxidative Factors
Samples (50 mL) were collected and centrifuged on incubation days 0, 3, 6, and 9. Before cell disruption, the resulting pellet was washed twice with HEPES buffer (50 mM, pH 7.0). Subsequently, it was placed in 2 mL cold lysis buffer (50 mM KH2PO4, pH 7.8, 0.1 mM EDTA, 0.5% Triton X − 100) and homogenised by transferring to tubes containing a half volume of 0.42–0.6mm glass beads (Sigma-Aldrich, St. Louis, USA) and disrupted for 5 min at 6.5 ms−1 using a FastPrep®-24 MP bead beater (Biomedicals, Santa Ana, USA) with pauses of 1 min every minute. The protein content of the pretreated biomass for assessing the specific activity of POX and GST was quantified using the Lowry method (Waterborg, 2009).
2.4.3 Determination of Growth and the Antioxidative Activity of Microalgae
Microalgae growth was monitored throughout the incubation period to assess the health of cultures and identify any inhibition or enhancement caused by the mixtures of antibiotics. The cell count was conducted using the Celeromics Technologies SL Micro Counter® (Valencia, Spain) under an optical microscope (Brunel Microscopes Ltd, Chippenham, UK) at a magnification of 20x. From the cell count, the growth rate was calculated following Eq. (1):
where μ is the growth rate per day, determined by taking the difference between the logarithmic cell number on day 9 and day 0, and then dividing this difference by the 9-day incubation period.
MDA content analysis followed a modified version of a previously reported protocol (Pancha et al., 2015). Briefly, 0.5 mL of homogenate was combined with an equal volume of 0.65% thiobarbituric acid (TBA) solution, prepared in a 20% (w/v) trichloroacetic acid (TCA) solution containing 0.01% butylated hydroxytoluene (BHT). The resulting mixture was subjected to heating at 95 °C for 20 min. After cooling to room temperature, the solution underwent centrifugation at 3,000 rpm for 10 min. The supernatant’s absorbance (A) was measured at 450 nm, 532 nm, and 600 nm using a Genesis 10S UV–Vis spectrophotometer (Thermo Scientific, Waltham, USA). The MDA content was determined using the following Eq. (2):
POX activity was assessed using the modified method by Baldev et al. (2013) by incubating 60 μL of homogenate in a solution containing hydrogen peroxide and potassium phosphate (0.05 mM, 0.1 M, pH 6.0), along with pyrogallol (2 mM L−1) in a mixture of sodium acetate (pH 4) and HEPES (100 mM, pH 7.0). The enzymatic assay was carried out in triplicate, and the absorbance changes were monitored at 450 nm, with readings taken every 30 s for 5 minutes in a microplate reader Agilent BioTek™ ELx808 (Santa Clara, USA).
GST activity was determined by the method of Cairrão et al. (2004) by monitoring changes in absorbance at 340 nm, indicative of the formation of the CDNB-GSH conjugate catalysed by GST. The reaction solution was prepared by combining 7.65 mL of sodium phosphate buffer (50 mM, pH 7.5), 250 μL of a 40 mM GSH solution in the phosphate buffer, and 100 μL of a 40 mM CDNB solution dissolved in ethanol 96%. Subsequently, 150 μL of this reaction mixture was added to 50 μL of the homogenate in a microplate. The enzymatic assay was carried out in triplicate, and absorbance measurements were recorded over 5 min at 30-s intervals using the microplate reader. GST and POX activities were quantified and expressed in nM mL−1 mg−1.
2.4.4 Determination of Nutrient Removal
The analysis of ammonium (NH4+-N) and phosphate (PO43−-P) ion concentrations in samples was conducted following established methods (Escudero et al., 2020). Ammonium ion concentrations were determined through a colourimetric method, following the Nessler protocol (Folin & Denis, 1916). The standard method 4500 P-E, as described by Baird and Bridgewater (2017), was employed to analyse phosphate ions.
2.5 Data Analysis
Statistical analysis was conducted using Microsoft Excel 2016 (Microsoft Corporation, Washington, USA) and SPSS Statistics V26.0 (IBM SPSS Statistics, Armonk, USA). To assess significant differences at confidence levels of 95% and 99% (p < 0.05 and 0.01), independent t-test, with variances equality tested using Levene’s Test, and one-way ANOVA were employed. Standard deviation values were computed and referenced based on a minimum of three data points.
3 Results and Discussion
3.1 Response Mechanisms
3.1.1 Microalgal Growth
In the present study, the growth of the algae exposed to the mixture of antibiotics was analysed by measuring the cell number over the incubation period (Fig. 1). The results showed that the antibiotic mixture impacted the growth of T. obliquus and C. acidophila, with a significant 26.6% and 11.6% growth inhibition (p < 0.05). For T. obliquus, the growth rate in the medium with antibiotics was 0.08 d−1, lower than that of the control group, 0.10 d−1, while for C. acidophila, the growth rates passed from 0.13 d−1 of the control to 0.11 d−1. On the other hand, A. protothecoides exhibited a significant increase (p < 0.01) in growth when exposed to antibiotics. During the 9-day cultivation period, the mixture caused a 30.6% increase in growth compared to the control, from 0.05 to 0.07 d−1. Generally, comparing the three strains, C. acidophila excelled, surpassing the other two strains in growth rate whether antibiotics were present or not.
The three microalgae species were generally affected by antibiotics in media exposed to high-stress scenarios, characterised by elevated antibiotic levels and low nutrient concentrations, often found in WW. Previous studies have also confirmed the inhibitory or stimulatory effects of antibiotics on microalgae growth. For instance, a prior study demonstrated that a mixture of antibiotics, including TMP, SMX, and CLA at 100 µg L−1, slowed the growth of Chlorella vulgaris, Scenedesmus quadricauda, and Raphidocelis subcapitata (Kiki et al., 2020). However, at a lower concentration (20 µg L−1) in the same study, the growth of Haematococcus pluvialis, S. quadricauda, and C. vulgaris increased significantly. Similarly, Chen et al. (2020) reported the dual effects of sulfonamides on C. vulgaris with stimulation up to 6% and inhibition up to 27%. In the present study, antibiotics inhibited the growth of some algae while stimulating the growth of others, particularly in the case of A. protothecoides. In particular, the enhancement of the algal growth can be attributed to hormesis, which can be explained in several ways. Firstly, low concentrations of antibiotics may enhance photosynthetic activity. Notably, recent research has shown that antibiotics at concentrations typically found in WWTPs induce stress responses, leading to an overproduction of pigments (Frascaroli et al., 2024). These pigments, particularly chlorophylls, may break down antibiotics or neutralise the ROS they generate (Luo et al., 2015). Moreover, the heightened production of pigments enhances light absorption, promoting algal photosynthesis and, thence, growth, as demonstrated in prior studies (Wan et al., 2021). The utilisation of antibiotics as a carbon source could also have contributed to the growth of A. protothecoides, a species known to thrive in mixotrophic conditions (Markou et al., 2021). Lastly, recent investigations have proposed that even low concentrations of antibiotics can upregulate specific genes and pathways related to DNA replication, leading to increased cell density (Jiang et al., 2021). The overall effectiveness of a microalgae-based system depends on the impact of pollutants like antibiotics on algal biomass. Increased growth may imply improved co-product production, while inhibition suggests reduced WW treatment capability. In-depth explorations of these effects and their correlation with productivity analysis are essential.
3.1.2 Nutrient Removal
The culture medium employed in this experimental study was explicitly formulated to emulate real-world nutrient concentrations found in WW, wherein ammonium (NH4+-N) and phosphate (PO43−-P) ions constitute two prominent inorganic pollutants. Following the 9-day incubation period, the three microalgae species exhibited varying capacities for pollutant removal, yielding moderate removal percentages ranging from 11 to 26% for ammonium and 15% to 42% for phosphate (Fig. 2). Among these species, T. obliquus emerged as the most proficient, demonstrating the ability to recover 1.67 − 1.78 mg L−1 day−1 of ammonium and 0.28–0.31 mg L−1 day−1 of phosphate. Conversely, C. acidophila exhibited more limited removal capabilities, achieving only 0.82 mg L−1 day−1 of ammonium and 0.17 mg L−1 day−1 of phosphate in the control conditions and 1.15 mg L−1 day−1 of ammonium and 0.13 mg L−1 day−1 of phosphate in the presence of antibiotics. The algae were solely responsible for nutrient removal, as the abiotic system had zero removal (data not shown). A stimulatory effect of antibiotics was also observed in this context, with a significant enhancement in ammonium removal by all microalgal species in the presence of antibiotics, especially by A. protothecoides (Fig. 2a). This led to removal efficiencies for this nutrient increasing by 13.9%, 28.8%, and 46.6% for T. obliquus, C. acidophila, and A. protothecoides, respectively. A similar trend was observed for phosphate removal only by A. protothecoides, which exhibited a 44.8% increase in antibiotics, from 1.0 to 1.8 mg L−1. In the presence of antibiotics, T. obliquus and C. acidophila exhibited a slight decrease in phosphate removal (0.3 and 0.4 mg L−1), although no statistically significant difference (p > 0.05) was observed between controls and treatments (Fig. 2b). Conversely, A. protothecoides demonstrated improved phosphate assimilation and increased growth (Fig. 1). The distinct nutrient assimilation patterns, especially for phosphate, underscore its role as a limiting factor affecting growth rates differently among the three species.
The identification of a stimulatory effect in this study underscores the complexity of nutrient removal by microalgae and the potential for response to external factors, including antibiotics. Recent studies have identified the dual hormetic role of pollutants found in WWTPs on the nutrient removal capabilities of microalgae, with lower concentrations enhancing algal metabolic activity and, thence, removal and higher doses typically causing inhibition (Atengueño-Reyes et al., 2023; Kiki et al., 2022; Zhou et al., 2016). In this context, it was noteworthy that antibiotics significantly enhanced ammonium recovery for all the algae and phosphate recovery specifically for A. protothecoides. Conversely, there was a slight decrease in phosphate uptake, which was not significant for the other two species. This suggests that antibiotics may have positive and adverse effects on the removal abilities of specific microalgae species. To the best of the authors’ knowledge, this is the first study to emphasise the stimulatory impact of antibiotics at concentrations found in WW on nutrient removal. This highlights the importance of further research to explore the molecular and cellular mechanisms underlying the enhancement of nutrient removal in microalgae due to antibiotics. Such investigations can aid in identifying suitable microalgal strains for efficient nutrient recovery without experiencing any detrimental effects from antibiotic exposure.
3.1.3 Antioxidative Activity
The results presented in Fig. 3 indicate a slight difference in the antioxidative activity between the controls and treatments within the microalgae. Following a 9-day exposure to the mixture, it was observed that only the POX activity (Fig. 3b) and MDA content (Fig. 3c) varied significantly for two of the three microalgae species. When exposed to a mixture of seven antibiotics at a concentration of 100 µg L−1, A. protothecoides and T. obliquus showed significant increases in POX activity. A. protothecoides had a 9.7-fold increase, while T. obliquus showed a 1.4-fold rise compared to their controls. C. acidophila also had a slight 1.1-fold increase in POX activity, but this change was not statistically significant (p > 0.05) (Fig. 3b). Furthermore, A. protothecoides exhibited a significant 20.3% decrease in MDA content following 9 days of exposure to the antibiotic mixture, while this parameter remained relatively stable for the other two algae species. Conversely, the GST activity remained relatively consistent across all three microalgae species assessed. In this case, it is worth noting that while slight increases in GST activity were observed in C. acidophila and A. protothecoides, these changes were not statistically significant (p > 0.05) (Fig. 3a).
In this study, it was observed that the content of GST increased in two of the algae after exposure to antibiotics, although the increase was not statistically significant (p > 0.05) (Fig. 3a). The primary function of GST is to detoxify substances like antibiotics by catalysing the conjugation of glutathione (GSH) to these compounds (Le et al., 2023). This process facilitates the removal of antibiotics, reducing their toxicity (Nie et al., 2009). A study from Guo et al. (2020) showed increased GST and GSH levels in R. subcapitata exposed to 40 μg L−1 of CLA, indicating their involvement in detoxifying antibiotics.
Antibiotics can also cause stress in microalgae by generating ROS, such as hydrogen peroxide, which usually accumulates in microalgal chloroplasts, mitochondria and peroxisomes (Gomaa et al., 2021). In this study, A. protothecoides and T. obliquus exhibited a notable elevation in POX antioxidants following antibiotic exposure. Conversely, C. acidophila displayed only a slight and statistically non-significant increase (p > 0.05), suggesting that the algae likely favoured alternative detoxifying mechanisms activated in the presence of low concentrations of stressors, such as pigments (Peng et al., 2021; Wan et al., 2021). POX enzymes are necessary to protect chloroplasts and other cell organelles from damage caused by hydrogen peroxide produced from them (Asada, 1992). Gomaa et al. (2021) observed a significant increase in ascorbate POX activity in Chlorella sp. and Desmodesmus spinosus exposed to antibiotics such as CIP, hypothesising the involvement of this enzyme in the primary reduction of ROS.
The excessive presence of ROS can lead to interactions with macromolecules, thereby initiating membrane lipid peroxidation (Aderemi et al., 2018). This process results in the release of MDA, recognised as one of the ultimate byproducts of lipid peroxidation. The antioxidative activity exhibited by microalgae, including the heightened activity of GST and POX and augmented production of pigments, collectively reinforces the cellular antioxidant capacity (Peng et al., 2021). In the context of the current investigation, a significant reduction in MDA content for A. protothecoides was observed, indicating a tangible contribution of the antioxidative enzymes to the mitigation of oxidative damage and the resultant decline in MDA content. In a recent study by Mao et al. (2021), the authors explored the impact of low concentrations of azithromycin on Chlorella pyrenoidosa. Interestingly, exposure to these low azithromycin concentrations (0.5 and 1 μg L−1) reduced oxidative damage, as evidenced by decreased MDA content. This reduction was closely associated with the augmentation of photosynthetic activities, attributable to an upregulation in the expression of psbA, a gene involved in the synthesis of photosystem II reaction centre and an enhanced synthesis of chlorophyll b and carotenoids. Therefore, the observed reduction of MDA appears to align with the concept of hormesis once again, where low levels of stressors confer beneficial effects.
The three algae displayed a marginal rise in antioxidant enzyme activity upon antibiotic exposure. However, this does not preclude the possibility that algae prefer alternative detoxifying mechanisms, like pigment overregulation, which may activate prior to the engagement of antioxidant factors. Long-term studies with additional variables relevant to actual WWTPs are essential to determine the enduring efficacy of the various complexity of antioxidant factors on the preservation of algal integrity, thereby evaluating the practical use of microalgae-based technologies to treat WW.
3.2 Antibiotic Removal
3.2.1 Abiotic Degradation
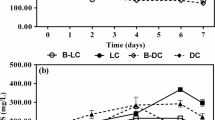
The abiotic degradation of the 7 antibiotics under two distinct pH conditions (3.2 and 7), involving photolysis and hydrolysis, was assessed. This evaluation included observing changes in antibiotic concentrations after 9 days in the water phase, with hydrolysis assessed using black nitrile-covered flasks and photolysis determined by comparing light-exposed and dark abiotic degradation. The findings revealed that abiotic degradation processes influenced 4 of the 7 antibiotics studied. Specifically, depending on the specific pH conditions, CIP, CLA, ERY, and OFL exhibited differential susceptibility to hydrolysis and photolysis (Fig. 4). On the other hand, TMP, SMX, and MDZ exhibited no abiotic degradation, aligning with their resistance to hydrolysis and photolysis, as previously reported (Bahman et al., 2022; Bai & Acharya, 2016; Lindberg et al., 2021). Notably, after 9 days at pH 3.2, CIP degradation was primarily driven by photolysis, with an efficiency of 78.4%. Conversely, degradation rates in the pH 7.0 media exceeded 80%, attributed principally to hydrolysis. The percentage of OFL degradation followed the same pattern, reaching 56.7% and 79.3% at pH 3.2 and 7.0, respectively, due to photolysis and hydrolysis. On the other hand, ERY and CLA exhibited distinct behaviour. At pH 3.2, these antibiotics primarily underwent degradation through hydrolysis, resulting in degradation percentages ranging from 70 to 96%. In contrast, at pH 7.0, their degradation was lower due to hydrolysis and photolysis, ranging from 34 to 49%.
These findings are consistent with existing literature. For example, in a study by Hom-Diaz et al. (2022) that employed a comparable system to distinguish between hydrolysis and photolysis, they observed an abiotic dark degradation – which we interpret as hydrolysis – of 7% for OFL and 29% for ERY, along with a photolysis process – referred to as photo-degradation by the authors – accounting for 78% and 5% degradation of OFL and ERY, respectively. From the results of this study, the extent to which photolysis and hydrolysis contribute to the abiotic degradation process is tied to the pH medium and the stability of the antibiotic at different pH levels. For instance, at pH 3.2 in the dark abiotic medium, the contribution of hydrolysis to CIP degradation was equal to zero. This behaviour can be elucidated by considering the chemical structure of CIP, which includes a piperazine ring and a carboxylic acid functional group (COOH). Notably, the highest stability of CIP is observed in solutions with a pH range of 3.0 to 4.0, where the COOH group remains non-ionised, and the basic nitrogen is fully protonated (National Center for Biotechnology Information, accessed 2024). Conversely, the photolysis of CIP was 4 times higher in low pH media when the flasks were exposed to light. The literature has previously reported pH-dependent photolysis for this antibiotic, with acidic conditions accelerating the photolysis process (Torniainen et al., 1996; Alabbas & Abdel-Gawad, 2023). The same principle can be extended to OFL, as its structure maintains the fundamental piperazine ring and COOH group of CIP. The molecular structures of the two antibiotics provide stability against hydrolysis while increasing their susceptibility to photolysis under low pH conditions.
In contrast, CLA and ERY exhibited a higher susceptibility to degradation driven by hydrolysis at pH 3.2. The pronounced and rapid degradation of ERY under acidic conditions in aqueous environments has been extensively documented in prior studies (Brisaert et al., 1996; Cachet et al., 1989; Cyphert et al., 2017). A similar trend is observed for CLA, with its half-life at pH 3 measured at 15.8 h (Erah et al., 1997), corroborating the almost total degradation observed here at pH 3.2 after 9 days. Our findings and existing literature indicate that rapid degradation primarily occurs for these two antibiotics, especially in acidic media, with photolysis playing a secondary role in media at a neutral pH.
3.2.2 Biosorption and Active Removal
Algal removal can be divided into three stages: initial rapid adsorption, followed by gradual bioaccumulation, and ultimately, biodegradation. Biosorption is an inert process that involves the bonding of antibiotics into microalgal cell surfaces (Kiki et al., 2020; Yu et al., 2017). In this study, microalgal biomass biosorption, quantified post-spiking and homogenisation, involved assessing the difference in antibiotic concentrations between the water phases of abiotic and biotic media at the start of the experiment (with a 10-min contact time). From the results, all antibiotics underwent rapid biosorption with variable efficiencies depending on the algae (Fig. 5). T. obliquus and C. acidophila exhibited important performance, passively removing 52.3% and 41.1% (492.1 and 398.5 µg L−1) of the total antibiotics immediately after spiking the mixture. Conversely, A. protothecoides displayed lower efficiency, adsorbing only 13.4% of the antibiotics (44.4 µg), with OFL and ERY showing higher adsorption rates at 37.2% and 51.5%, respectively. T. obliquus excelled in removing CIP (91.5%) and OFL (73.5%), as did C. acidophila (82.4% and 60.0%), highlighting the influence of the similar chemical structures of these two antibiotics on biosorption efficiency. A comparable biosorption pattern was evident for T. obliquus and C. acidophila when it came to TMP and MDZ, with removal rates of 24% and 14–21%, respectively. Conversely, all three microalgae exhibited similar removal rates for ERY, ranging from 52 to 58%. It is also important to highlight that SMX exhibited the highest resistance to adsorption among the antibiotics studied. T. obliquus was the sole species capable of removing it (50.5%).
Although the phenomenon of biosorption for the antibiotics studied in this study is not new in the literature, to the authors’ knowledge, this is the first time such high percentages of removal were reported. Prior research had noted lower removal rates, such as 5% for CIP using Chlamydomonas sp. Tai-03 (Xie et al., 2020) or 2% for SMX and 13% for TMP using C. vulgaris (Kiki et al., 2020). Various microalgae strains, including those from Chlorella, Coelastrella sp., Desmodesmus sp., and Scenedesmus sp., showed negligible OFL and TMP adsorption when exposed to a medium containing a mixture of nineteen pharmaceuticals (Gojkovic et al., 2019). On the other hand, MDZ adsorption percentages exceeding 80% have been reported when employing live Synechocystis sp. cells or exhausted biomass from Spirulina platensis (Bahman et al., 2022; Esmaili et al., 2023).
The variation and the similarities in biosorption rates among the three species employed in this study could be attributed to their phylogenetic relationships. Specifically, A. protothecoides is classified under the Trebouxiophyceae class, while T. obliquus and C. acidophila belong to the Chlorophyceae class (Schoch, et al. 2020). Therefore, certain resemblances in the cell wall compositions of Scenedesmus sp. and Chlamydomonas sp. distinguish them from A. protothecoides. In a comprehensive analysis conducted by Baudelet et al. (2017), which delved deeply into the cell wall structure and composition of the Chlorophyta, it emerges that Scenedesmus sp. and Chlamydomonas sp. share a common feature: both of them possess algaenan, a biopolymer found within their cell walls providing integrity to the microalgae cell walls. Interestingly, this substance does not appear to be a component of the cell wall in A. protothecoides, although it has been observed in other species belonging to the Trebouxiophyceae class. Algaenan comprises aliphatic chains, alcohols, fatty acids, and dicarboxylic acids interconnected by ester and ether linkages. All of these compounds, with their functional groups, may play a pivotal role in antibiotic adsorption and the formation of chemical bonds. Additionally, it was noted that various members of the Chlorellaceae family, to which A. protothecoides belong, generate a matrix made of extracellular polymeric substances around their walls to facilitate their interactions with fungal and bacterial symbionts (Baudelet et al., 2017). These substances could potentially negatively affect the absorption of antibiotics by creating a barrier that hinders the chemical interactions between cell walls and antibiotics. It is also important to note that the use of two different pH values, 3.2 and 7, could have influenced the efficiency of biosorption. Adsorption of compounds strongly depends on pH, as it affects the charge of both microalgae surfaces and functional groups of the antibiotics based on their pKa values. These groups can become positively or negatively charged through protonation or deprotonation, influenced by the pH of the solution. When the pH is below the pH of zero charge (pHPZC, where the algal surface has no net electrical charge), the microalgae biomass carries a positive surface charge, favouring the biosorption of negatively charged antibiotics. Conversely, at pH values higher than pHPZC, the surface charge becomes negative, promoting the biosorption of positively charged antibiotics (Silva et al., 2020). In this study, for instance, T. obliquus was the only species absorbing SMX. SMX exhibits three forms (protonated, non-protonated, and deprotonated) at varying pH values. At pH below 1.6, SMX is protonated; at pH 3.0, it becomes non-protonated; and at pH 7.0, it is deprotonated, as hydrogen dissociates from the sulfonamide group when pH exceeds 5.7 (Oyekunle et al., 2022). The higher reactivity of deprotonated SMX enhances its removal, but the surface must be receptive for effective bonding with microalgae. This, linked to what was described earlier on the production of extracellular polymeric substances by Chlorophyceae, could explain why we did not observe absorption in A. protothecoides. Considering the quick and effective biosorption illustrated in this study, there is potential for advancing the application of algae as a prompt and efficient approach for eliminating antibiotics from WW. This is especially significant in addressing AMR, as the capability to rapidly and efficiently eliminate antibiotics from WW may reduce the environmental exposure of these substances to bacteria. Furthermore, the high biosorption percentages demonstrated in this study raise questions about the potential reusability of a biomass enriched with antibiotics following WW treatment.
If biosorption occurs during the initial phases of microalgae interaction with antibiotics, active removal, an energy-dependent process including bioaccumulation and biodegradation, comes into play at later stages (Kiki et al., 2020; Yu et al., 2017). In this study, active removal was evaluated by quantifying antibiotic concentration reduction in the water phase, comparing levels post-initial biosorption with those after 9 days in the media with algae. Active removal occurred for all the antibiotics but differently depending on the algae studied (Fig. 6 and Table S6 in Supplementary Information). Among the three studied microalgae, A. protothecoides demonstrated important capability, removing 22.1% of the total remaining antibiotics after initial biosorption. Notably, it eliminated 67.6%, 51.7%, 24.8%, and 10.5% of SMX, CIP, MDZ, and TMP, respectively (Fig. 6a). In contrast, C. acidophila exhibited proficiency by eliminating a residual 16.3% of the total antibiotics, showcasing particular efficacy in removing CLA and ERY. Notably, 76.0% and 25.9% removal were observed over time for the two antibiotics, primarily attributed to the acidic medium facilitating the hydrolytic breakdown of these compounds in the water phase. Furthermore, this species exhibited removal capabilities for SMX (23.4%) (Fig. 6b). Lastly, T. obliquus showed comparatively lower removal rates for certain antibiotics, including OFL, CIP and SMX, with removal efficiencies of 3.7%, 11.2% and 7.3%, respectively (Fig. 6c) and overall active removal of 3.2% (Supplementary Information, Table S6).
Active removal through accumulation and degradation has been documented in previous studies. Kiki et al. (2020) demonstrated a biodegradation range of 25% to 95% for CLA and SMX using four microalgae species in synthetic WW. Ding et al. (2020) reported slight bioaccumulation of OFL using the freshwater diatom Navicula sp. Additionally, Gojkovic et al. (2019) observed active removal efficiencies of 20% for TMP by T. obliquus in a standard medium. Based on the presented results, it is evident that the active removal process is highly dependent on the specific microalga and antibiotic being used, as certain ones are more efficiently removed than others. Notably, all three microalgae successfully removed SMX over time, which has been extensively researched in previous studies (Xiong et al., 2019; Zhang et al., 2022). Recent research by Chu et al. (2022) emphasised the involvement of cytochrome P450 monooxygenase in intracellular SMX degradation, which occurs through hydroxylation of the aniline ring or dihydroxylation of the isoxazole ring, followed by ring opening and direct cleavage of the sulfonamide bond. Moreover, of particular note is the pattern of CIP concerning C. acidophila, which exhibited a continuous increase in concentration during incubation (Fig. 6b). Initially, at day 0, the CIP concentration in the medium was 30.0 µg L−1, eventually reaching a value of 49.1 µg L−1 after 9 days. This trend suggests a consistent antibiotic release by the microalga over time, with approximately 11.1% of the initially adsorbed antibiotic gradually desorbed. The release of antibiotics has been demonstrated in earlier studies (Chen et al., 2020; Frascaroli et al., 2024), and the pH of the medium strongly influences it. In the experiment involving C. acidophila, at the pH of 3.2 employed, CIP exists in a protonated state, potentially leading to initial passive adsorption by the biomass and continuous release due to the tendency of the antibiotic to dissolve in the acidic medium.
3.2.3 Total Removal
In conclusion, the comparison of abiotic and biotic removal, encompassing both biosorption and active removal, of antibiotics over 9 days distinctly highlights the predominant impact of the acidic environment on the degradation of specific antibiotics, such as CLA and ERY (Table 2). In the abiotic and biotic media, where the pH of the medium was 3.2, both antibiotics exhibited similar levels of removal, regardless of the presence of C. acidophila, suggesting that the primary factor driving removal is the hydrolytic degradation caused by the low pH of the medium. Furthermore, the light-shading effect of microalgae, which led to reduced removal of specific antibiotics, was also observed. This effect was particularly notable for CIP and OFL in the presence of A. protothecoides, resulting in a lower removal percentage than the abiotic control, where photolysis was mainly responsible for the degradation of both antibiotics (Table 2 and Fig. 4). On the other hand, the shielding effect potentially generated by the algae biomass may have been a crucial factor in diminishing the hydrolytic degradation of CLA and ERY at pH 7.0, considering the previously observed bioabsorption of these antibiotics ranging from 14–34% and 52–58%, respectively (Fig. 5 and Table S6 in Supplementary Information). The shading effect resulting from the algal biomass, which leads to a decrease in abiotic degradation of specific antibiotics, has been documented in previous studies (de Godos et al., 2012; Kiki et al., 2020).
In summary, these results emphasise the varying abilities of the 3 microalgae species in removing antibiotics, potentially reducing the impact of AMR in the aquatic environment. A. protothecoides demonstrated effectiveness in removing actively certain antibiotics, whereas T. obliquus exhibited higher removal capacities for the antibiotics studied in terms of biosorption. Conversely, the low pH of the medium impreoved the removal performance of C. acidophila, suggesting that low pH conditions could potentially be employed for antibiotic and pollutant removal purposes. Future research could explore strategies to optimise microalgae-based antibiotic removal based on abiotic and biotic factors, considering the effect of pH and the light-shading effects observed in this study. This might include optimisation of microalgae strains with reduced shading or shielding capabilities or adjusting WW pH values and WWTPs design to maximise hydrolytic and photolytic degradation.
4 Conclusions
Rapid biosorption was achieved employing T. obliquus and C. acidophila, with influences from antibiotic structures and the pH of the media. This suggests potential approaches for the quick removal of specific commonly used antibiotics. The active removal of selected antibiotics over time, especially for A. protothecoides, demonstrated that extending the contact time in WW could significantly enhance the overall removal process. Antibiotic removal by C. acidophila was pH-dependent, with enhanced degradation under acidic conditions, particularly for clarithromycin and erythromycin. Certain antibiotics, like ciprofloxacin and ofloxacin, were susceptible to abiotic hydrolysis and pH-dependent photolysis, suggesting potential improvement in removal by manipulating WW pH conditions.
A stimulatory effect was observed, as antibiotics notably improved ammonium and phosphate removal across all species, especially in A. protothecoides, marking an initial demonstration of their potential impact on nutrient removal in WW treatment. Distinct antioxidative activities were observed on a species-specific basis, indicating that algae employ varying mechanisms to mitigate antibiotics-induced stress. A. protothecoides showed increased growth and reduced MDA in the presence of antibiotics, confirming the stimulatory effect of these pollutants at concentrations found in WW.
The three microalgae species removed antibiotics, with A. protothecoides showing particular resistance to high antibiotics in conditions similar to real WW. Implementing these microalgae in WW treatment could significantly mitigate the presence of antibiotics and the impact of AMR in aquatic environments.
Data Availability
Data will be made available on request.
References
Aderemi, A. O., Novais, S. C., Lemos, M. F., Alves, L. M., Hunter, C., & Pahl, O. (2018). Oxidative stress responses and cellular energy allocation changes in microalgae following exposure to widely used human antibiotics. Aquatic Toxicology, 203, 130–139. https://doi.org/10.1016/j.aquatox.2018.08.008
Alabbas, A. B., & Abdel-Gawad, A. S. (2023). Stability-Indicating Quantification of Ciprofloxacin in the Presence of Its Main Photodegradation Product by CZE and UPLC: A Comparative Study. Separations, 10(7), 391. https://doi.org/10.3390/separations10070391
Ali, M. E., Abd El-Aty, A. M., Badawy, M. I., & Ali, R. K. (2018). Removal of pharmaceutical pollutants from synthetic wastewater using chemically modified biomass of green alga Scenedesmus obliquus. Ecotoxicology and Environmental Safety, 151, 144–152. https://doi.org/10.1016/j.ecoenv.2018.01.012
Antimicrobial Resistance Collaborators. (2022). Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet, 399(10325), 629–655. https://doi.org/10.1016/S0140-6736(21)02724-0
Asada, K. (1992). Ascorbate peroxidase – a hydrogen peroxide-scavenging enzyme in plants. Physiologia Plantarum, 85(2), 235–241. https://doi.org/10.1111/j.1399-3054.1992.tb04728.x
Atengueño-Reyes, K., Velasquez-Orta, S. B., Yáñez-Noguez, I., Monje-Ramirez, I., Mijaylova-Nacheva, P., Chávez-Mejía, A., & Orta Ledesma, M. (2023). Microalgal consortium tolerance to bisphenol A and triclosan in wastewater and their effects on growth, biomolecule content and nutrient removal. Ecotoxicology and Environmental Safety, 262, 115117. https://doi.org/10.1016/j.ecoenv.2023.115117
Ayala, A., Muñoz, M. F., & Argüelles, S. (2014). Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Medicine and Cellular Longevity, 360438. https://doi.org/10.1155/2014/360438
Bahman, M., Jalili, H., Etesam, M., & Amrane, A. (2022). Investigation of pharmaceutical compounds (Metronidazole, Rosuvastatin and Codeine phosphate) removal by Synechocystis sp. PCC6803 microalga. Journal of Water Process Engineering, 47, 102820. https://doi.org/10.1016/j.jwpe.2022.102820
Bai, X., & Acharya, K. (2016). Removal of trimethoprim, sulfamethoxazole, and triclosan by the green alga Nannochloris sp. Journal of Hazardous Materials, 315, 70–75. https://doi.org/10.1016/j.jhazmat.2016.04.067
Baird, R., & Bridgewater, L. (2017). Standard methods for the examination of water and wastewater. 23rd edition. Washington, D.C., American Public Health Association.
Baldev, E., MubarakAli, D., Ilavarasi, A., Pandiaraj, D., Ishack, K. S. S., & Thajuddin, N. (2013). Degradation of synthetic dye, Rhodamine B to environmentally non-toxic products using microalgae. Colloids and Surfaces B, 105, 207–214. https://doi.org/10.1016/j.colsurfb.2013.01.008
Baudelet, P., Ricochon, G., Linder, M., & Muniglia, L. (2017). A new insight into cell walls of Chlorophyta. Algal Research, 25, 333–371. https://doi.org/10.1016/j.algal.2017.04.008
Brisaert, M., Heylen, M., & Plaizier-Vercammen, J. (1996). Investigation on the chemical stability of erythromycin in solutions using an optimisation system. Pharmacy World and Science, 18, 182–186. https://doi.org/10.1007/BF00820730
Cachet, T., Van den Mooter, G., Hauchecorne, R., Vinckier, C., & Hoogmartens, J. (1989). Decomposition kinetics of erythromycin A in acidic aqueous solutions. International Journal of Pharmaceutics, 55(1), 59–65. https://doi.org/10.1016/0378-5173(89)90277-9
Cairrão, E., Couderchet, M., Soares, A., & Guilhermino, L. (2004). Glutathione-S-transferase activity of Fucus spp. As a biomarker of environmental contamination. Aquatic Toxicology, 70(4), 277–286. https://doi.org/10.1016/j.aquatox.2004.09.005
Chen, S., Zhang, W., Li, J., Yuan, M., Zhang, J., Xu, F., Xu, H., Zheng, X., & Wang, L. (2020). Ecotoxicological effects of sulfonamides and fluoroquinolones and their removal by a green alga (Chlorella vulgaris) and a cyanobacterium (Chrysosporum ovalisporum). Environmental Pollution, 263, 114554. https://doi.org/10.1016/j.envpol.2020.114554
Chu, Y., Zhang, C., Wang, R., Chen, X., Ren, N., & Ho, S. (2022). Biotransformation of sulfamethoxazole by microalgae: Removal efficiency, pathways, and mechanisms. Water Research, 221, 118834. https://doi.org/10.1016/j.watres.2022.118834
Cyphert, E. L., Wallat, J. D., Pokorski, J. K., & Von Recum, H. A. (2017). Erythromycin Modification That Improves Its Acidic Stability while Optimising It for Local Drug Delivery. Antibiotics, 6(2), 11. https://doi.org/10.3390/antibiotics6020011
Dadgostar, P. (2019). Antimicrobial Resistance: Implications and Costs. Infection and Drug Resistance, 12, 3903–3910. https://doi.org/10.2147/IDR.S234610
de Godos, I., Muñoz, R., & Guieysse, B. (2012). Tetracycline removal during WW treatment in high-rate algal ponds. Journal of Hazardous Materials, 229–230, 446–449. https://doi.org/10.1016/j.jhazmat.2012.05.106
Ding, T., Wang, S., Yang, B., & Li, J. (2020). Biological removal of pharmaceuticals by Navicula sp. And Biotransformation of Bezafibrate. Chemosphere, 240, 124949. https://doi.org/10.1016/j.chemosphere.2019.124949
Erah, P. O., Goddard, A. F., Barrett, D. A., Shaw, P. N., & Spiller, R. C. (1997). The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: Relevance to the treatment of Helicobacter pylori infection. Journal of Antimicrobial Chemotherapy, 39(1), 5–12. https://doi.org/10.1093/jac/39.1.5
Escudero, A., Hunter, C., Roberts, J., Helwig, K., & Pahl, O. (2020). Pharmaceuticals removal and nutrient recovery from wastewaters by Chlamydomonas acidophila. Biochemical Engineering Journal, 156, 107517. https://doi.org/10.1016/j.bej.2020.107517
Esmaili, Z., Barikbin, B., Shams, M., Alidadi, H., Al-Musawi, T. J., & Bonyadi, Z. (2023). Biosorption of metronidazole using Spirulina platensis microalgae: process modeling, kinetic, thermodynamic, and isotherm studies. Applied Water Science, 13(2), 63. https://doi.org/10.1007/s13201-023-01867-9
Folin, O., & Denis, W. (1916). Nitrogen determinations by direct nesslerisation: I Total nitrogen in urine. Journal of Biological Chemistry, 26(2), 473–489. https://doi.org/10.1016/S0021-9258(18)87427-0
Frascaroli, G., Roberts, J., Hunter, C., & Escudero, A. (2024). Removal efficiencies of seven frequently detected antibiotics and related physiological responses in three microalgae species. Environmental Science and Pollution Research, 1−13. https://doi.org/10.1007/s11356-024-32026-5
Frascaroli, G., Reid, D., Hunter, C., Roberts, J., Helwig, K., Spencer, J., & Escudero, A. (2021). Pharmaceuticals in Wastewater Treatment Plants: A Systematic Review on the Substances of Greatest Concern Responsible for the Development of Antimicrobial Resistance. Applied Sciences, 11(15), 6670. https://doi.org/10.3390/app11156670
Gojkovic, Z., Lindberg, R. H., Tysklind, M., & Funk, C. (2019). Northern green algae have the capacity to remove active pharmaceutical ingredients. Ecotoxicology and Environmental Safety, 170, 644–656. https://doi.org/10.1016/j.ecoenv.2018.12.032
Gomaa, M., Zien-Elabdeen, A., Hifney, A. F., & Adam, M. S. (2021). Phycotoxicity of antibiotics and non-steroidal anti-inflammatory drugs to green algae Chlorella sp. And Desmodesmus spinosus: Assessment of combined toxicity by Box-Behnken experimental design. Environmental Technology & Innovation, 23, 101586. https://doi.org/10.1016/j.eti.2021.101586
Guo, J., Peng, J., Lei, Y., Kanerva, M., Li, Q., Song, J., Guo, J., & Sun, H. (2020). Comparison of oxidative stress induced by clarithromycin in two freshwater microalgae Raphidocelis subcapitata and Chlorella vulgaris. Aquatic Toxicology, 219, 105376. https://doi.org/10.1016/j.aquatox.2019.105376
Hom-Diaz, A., Jaén-Gil, A., Rodríguez-Mozaz, S., Barceló, D., Vicent, T., & Blánquez, P. (2022). Insights into removal of antibiotics by selected microalgae (Chlamydomonas reinhardtii, Chlorella sorokiniana, Dunaliella tertiolecta and Pseudokirchneriella subcapitata). Algal Research, 61, 102560. https://doi.org/10.1016/j.algal.2021.102560
Huang, W., Li, B., Zhang, C., Zhang, Z., Lei, Z., Lu, B., & Zhou, B. (2015). Effect of algae growth on aerobic granulation and nutrients removal from synthetic wastewater by using sequencing batch reactors. Bioresource Technology, 179, 187–192. https://doi.org/10.1016/j.biortech.2014.12.024
Ji, B., Zhang, M., Gu, J., Ma, Y., & Liu, Y. (2020). A self-sustaining synergetic microalgal-bacterial granular sludge process towards energy-efficient and environmentally sustainable municipal wastewater treatment. Water Research, 179, 115884. https://doi.org/10.1016/j.watres.2020.115884
Jiang, Y., Liu, Y., & Zhang, J. (2021). Mechanisms for the stimulatory effects of a five-component mixture of antibiotics in Microcystis aeruginosa at transcriptomic and proteomic levels. Journal of Hazardous Materials, 406, 124722. https://doi.org/10.1016/j.jhazmat.2020.124722
Jones, E. R., van Vliet, M. T., Qadir, M., & Bierkens, M. F. (2021). Country-level and gridded estimates of wastewater production, collection, treatment and reuse. Earth System Science Data, 13(2), 237–254. https://doi.org/10.5194/essd-2020-156
Kiki, C., Rashid, A., Wang, Y., Li, Y., Zeng, Q., Yu, C., & Sun, Q. (2020). Dissipation of antibiotics by microalgae: Kinetics, identification of transformation products and pathways. Journal of Hazardous Materials, 387, 121985. https://doi.org/10.1016/j.jhazmat.2019.121985
Kiki, C., Rashid, A., Zhang, Y., Li, X., Chen, T., Eloise Adéoye, A. B., Peter, P. O., & Sun, Q. (2022). Microalgal mediated antibiotic co-metabolism: Kinetics, transformation products and pathways. Chemosphere, 292, 133438. https://doi.org/10.1016/j.chemosphere.2021.133438
Kotoula, D., Iliopoulou, A., Irakleous-Palaiologou, E., Gatidou, G., Aloupi, M., Antonopoulou, P., Fountoulakis, M. S., & Stasinakis, A. S. (2020). Municipal wastewater treatment by combining in series microalgae Chlorella sorokiniana and macrophyte Lemna minor: Preliminary results. Journal of Cleaner Production, 271, 122704. https://doi.org/10.1016/j.jclepro.2020.122704
Larsson, D. G., & Flach, C. (2022). Antibiotic resistance in the environment. Nature Reviews Microbiology, 20(5), 257–269. https://doi.org/10.1038/s41579-021-00649-x
Leng, L., Wei, L., Xiong, Q., Xu, S., Li, W., Lv, S., Lu, Q., Wan, L., Wen, Z., & Zhou, W. (2020). Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere, 238, 124680. https://doi.org/10.1016/j.chemosphere.2019.124680
Le, V. V., Tran, Q. G., Ko, S. R., Lee, S. A., Oh, H. M., Kim, H. S., & Ahn, C. Y. (2023). How do freshwater microalgae and cyanobacteria respond to antibiotics?. Critical Reviews in Biotechnology, 43(2), 191–211. https://doi.org/10.1080/07388551.2022.2026870
Li, K., Liu, Q., Fang, F., Luo, R., Lu, Q., Zhou, W., Huo, S., Cheng, P., Liu, J., Addy, M., Chen, P., Chen, D., & Ruan, R. (2019). Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresource Technology, 291, 121934. https://doi.org/10.1016/j.biortech.2019.121934
Lindberg, R. H., Namazkar, S., Lage, S., Östman, M., Gojkovic, Z., Funk, C., Gentili, F. G., & Tysklind, M. (2021). Fate of active pharmaceutical ingredients in a northern high-rate algal pond fed with municipal wastewater. Chemosphere, 271, 129763. https://doi.org/10.1016/j.chemosphere.2021.129763
Luo, L., Lai, X., Chen, B., Lin, L., Fang, L., Tam, N. F., & Luan, T. (2015). Chlorophyll catalyse the photo-transformation of carcinogenic benzo [a] pyrene in water. Science and Reports, 5, 1–11. https://doi.org/10.1038/srep12776
Manzi, H. P., Zhang, P., Zhang, L., Xing, X., Yue, J., Song, Z., Nan, L., Yujun, S., Khan, A., Yoon, Y., & Salama, E. (2022). Effect of dibutyl phthalate on microalgal growth kinetics, nutrients removal, and stress enzyme activities. Marine Environment Research, 181, 105741. https://doi.org/10.1016/j.marenvres.2022.105741
Mao, Y., Yu, Y., Ma, Z., Li, H., Yu, W., Cao, L., & He, Q. (2021). Azithromycin induces dual effects on microalgae: Roles of photosynthetic damage and oxidative stress. Ecotoxicology and Environmental Safety, 222, 112496. https://doi.org/10.1016/j.ecoenv.2021.112496
Markou, G., Diamantis, A., Korozi, E., Tsagou, V., Kefalogianni, I., & Chatzipavlidis, I. (2021). Effects of Monochromatic Illumination with LEDs Lights on the Growth and Photosynthetic Performance of Auxenochlorella protothecoides in Photo-and Mixotrophic Conditions. Plants, 10, 799. https://doi.org/10.3390/plants10040799
Maryjoseph, S., & Ketheesan, B. (2020). Microalgae based wastewater treatment for the removal of emerging contaminants: A review of challenges and opportunities. Case Studies in Chemical and Environmental Engineering, 2, 100046. https://doi.org/10.1016/j.cscee.2020.100046
National Center for Biotechnology Information. (2024). PubChem compound summary for CID 2764, Ciprofloxacin. Retrieved September 7, 2023, from https://pubchem.ncbi.nlm.nih.gov/compound/Ciprofloxacin
Negoro, K. (1944). Untersuchungen tiber die Vegetation der mineralogen-azidotrophen Gewassern Japans. Science and Reports, 101, 322–350.
Nie, X., Gu, J., Lu, J., Pan, W., & Yang, Y. (2009). Effects of norfloxacin and butylated hydroxyanisole on the freshwater microalga Scenedesmus obliquus. Ecotoxicology, 18, 677–684. https://doi.org/10.1007/s10646-009-0334-1
O’Neill, J. (2016). Tackling drug-resistant infections globally: final report and recommendations. Wellcome Trust and HM Government. https://apo.org.au/node/63983
Oyekunle, D. T., Gendy, E. A., Ifthikar, J., & Chen, Z. (2022). Heterogeneous activation of persulfate by metal and non-metal catalyst for the degradation of sulfamethoxazole: A review. Journal of Chemical Engineering, 437, 135277. https://doi.org/10.1016/j.cej.2022.135277
Pancha, I., Chokshi, K., Maurya, R., Trivedi, K., Patidar, S. K., Ghosh, A., & Mishra, S. (2015). Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresource Technology, 189, 341–348. https://doi.org/10.1016/j.biortech.2015.04.017
Peng, J., Guo, J., Lei, Y., Mo, J., Sun, H., & Song, J. (2021). Integrative analyses of transcriptomics and metabolomics in Raphidocelis subcapitata treated with clarithromycin. Chemosphere, 266, 128933. https://doi.org/10.1016/j.chemosphere.2020.128933
Preisner, M., Neverova-Dziopak, E., & Kowalewski, Z. (2020). Analysis of eutrophication potential of municipal wastewater. Water Science and Technology, 81(9), 1994–2003. https://doi.org/10.2166/wst.2020.254
Qv, M., Dai, D., Liu, D., Wu, Q., Tang, C., Li, S., & Zhu, L. (2023). Towards advanced nutrient removal by microalgae-bacteria symbiosis system for wastewater treatment. Bioresource Technology, 370, 128574. https://doi.org/10.1016/j.biortech.2022.128574
Schoch, C. L., et al. (2020). NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford), 2020, baaa062.
Silva, A., Coimbra, R. N., Escapa, C., Figueiredo, S. A., Freitas, O. M., & Otero, M. (2020). Green microalgae Scenedesmus obliquus utilization for the adsorptive removal of nonsteroidal anti-inflammatory drugs (NSAIDs) from water samples. International Journal of Environmental Research and Public Health, 17(10), 3707. https://doi.org/10.3390/ijerph17103707
Torniainen, K., Tammilehto, S., & Ulvi, V. (1996). The effect of pH, buffer type and drug concentration on the photodegradation of ciprofloxacin. International Journal of Pharmaceutics, 132(1–2), 53–61. https://doi.org/10.1016/0378-5173(95)04332-2
Tran, N. H., Chen, H., Reinhard, M., Mao, F., & Gin, K. Y. H. (2016). Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Research, 104, 461–472. https://doi.org/10.1016/j.watres.2016.08.040
Vassalle, L., Sunyer-Caldú, A., Díaz-Cruz, M. S., Arashiro, L. T., Ferrer, I., Garfí, M., & García-Galán, M. J. (2020). Behavior of UV filters, UV blockers and pharmaceuticals in high rate algal ponds treating urban wastewater. Water, 12, 2658. https://doi.org/10.3390/w12102658
Walls, L. E., Velasquez-Orta, S. B., Romero-Frasca, E., Leary, P., Yáñez Noguez, I., & Orta Ledesma, M. T. (2019). Non-sterile heterotrophic cultivation of native wastewater yeast and microalgae for integrated municipal wastewater treatment and bioethanol production. Biochemical Engineering Journal, 151, 107319. https://doi.org/10.1016/j.bej.2019.107319
Wan, L., Wu, Y., Zhang, B., Yang, W., Ding, H., & Zhang, W. (2021). Effects of moxifloxacin and gatifloxacin stress on growth, photosynthesis, antioxidant responses, and microcystin release in Microcystis aeruginosa. Journal of Hazardous Materials, 409, 124518. https://doi.org/10.1016/j.jhazmat.2020.124518
Wang, S., Ji, B., Zhang, M., Gu, J., Ma, Y., & Liu, Y. (2021). Tetracycline-induced decoupling of symbiosis in microalgal-bacterial granular sludge. Environmental Research, 197, 111095. https://doi.org/10.1016/j.envres.2021.111095
Waterborg, J.H. (2009). The Lowry Method for Protein Quantitation. In: Walker, J.M. (eds) The Protein Protocols Handbook. Springer Protocols Handbooks. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-59745-198-7_2
Xie, P., Chen, C., Zhang, C., Su, G., Ren, N., & Ho, S. (2020). Revealing the role of adsorption in ciprofloxacin and sulfadiazine elimination routes in microalgae. Water Research, 172, 115475. https://doi.org/10.1016/j.watres.2020.115475
Xiong, J., Govindwar, S., Kurade, M. B., Paeng, K., Roh, H., Khan, M. A., & Jeon, B. (2019). Toxicity of sulfamethazine and sulfamethoxazole and their removal by a green microalga, Scenedesmus obliquus. Chemosphere, 218, 551–558. https://doi.org/10.1016/j.chemosphere.2018.11.146
Xu, S., Liu, Y., & Zhang, J. (2022). Transcriptomic mechanisms for the promotion of cyanobacterial growth against eukaryotic microalgae by a ternary antibiotic mixture. Environmental Science and Pollution Research, 29(39), 58881–58891. https://doi.org/10.1007/s11356-022-20041-3
Yang, Q., Gao, Y., Ke, J., Show, P. L., Ge, Y., Liu, Y., Guo, R., & Chen, J. (2021). Antibiotics: An overview on the environmental occurrence, toxicity, degradation, and removal methods. Bioengineered, 12(1), 7376–7416. https://doi.org/10.1080/21655979.2021.1974657
Yu, Y., Zhou, Y., Wang, Z., Torres, O. L., Guo, R., & Chen, J. (2017). Investigation of the removal mechanism of antibiotic ceftazidime by green algae and subsequent microbic impact assessment. Science and Reports, 7(1), 1–11. https://doi.org/10.1038/s41598-017-04128-3
Zhang, Y., He, D., Chang, F., Dang, C., & Fu, J. (2021). Combined effects of sulfamethoxazole and erythromycin on a freshwater microalga, Raphidocelis subcapitata: Toxicity and oxidative stress. Antibiotics, 10(5), 576. https://doi.org/10.3390/antibiotics10050576
Zhang, Y., Wan, J., Li, Z., Wu, Z., Dang, C., & Fu, J. (2022). Enhanced removal efficiency of sulfamethoxazole by acclimated microalgae: Tolerant mechanism, and transformation products and pathways. Bioresource Technology, 347, 126461. https://doi.org/10.1016/j.biortech.2021.126461
Zhou, Q., Li, F., Ge, F., Liu, N., & Kuang, Y. (2016). Nutrient removal by Chlorella vulgaris F1068 under cetyltrimethyl ammonium bromide induced hormesis. Environmental Science and Pollution Research, 23, 19450–19460. https://doi.org/10.1007/s11356-016-6999-0
Zhou, W., Li, Y., Min, M., Hu, B., Zhang, H., Ma, X., Li, L., Cheng, Y., Chen, P., & Ruan, R. (2012). Growing wastewater-born microalga Auxenochlorella protothecoides UMN280 on concentrated municipal wastewater for simultaneous nutrient removal and energy feedstock production. Applied Energy, 98, 433–440. https://doi.org/10.1016/j.apenergy.2012.04.005
Acknowledgements
No funding was received to assist with the preparation of this manuscript. A heartfelt expression of gratitude is extended to Fra Brigasca, who was present throughout every stage of mental processing, experimental procedures, and writing. Finally, appreciation is extended to Crozzala VonLilif for the valuable mathematical and theoretical support provided during the revision.
Author information
Authors and Affiliations
Contributions
Conceptualisation Frascaroli; Data curation Frascaroli; Formal analysis Frascaroli; Funding acquisition Frascaroli and A.E; Investigation Frascaroli; Methodology Frascaroli; Project administration Frascaroli, Roberts, Hunter and Escudero; Resources Frascaroli, Roberts, Hunter and Escudero; Software Frascaroli; Supervision Roberts, Hunter and Escudero; Validation Frascaroli, Roberts, Hunter and Escudero; Visualization Frascaroli; Roles/Writing—original draft Frascaroli; Writing—review & editing Frascaroli, Roberts, Hunter and Escudero. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frascaroli, G., Hunter, C., Roberts, J. et al. Antibiotic Removal by Three Promising Microalgae Strains: Biotic, Abiotic Routes, and Response Mechanisms. Water Air Soil Pollut 235, 600 (2024). https://doi.org/10.1007/s11270-024-07385-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07385-x